Global State of Pain Treatment
Access to Palliative Care as a Human Right
Key Terms in Palliative Care and Pain Treatment
Essential medicine: A medicine included in the World Health Organization’s Model List of Essential Medicines.
Palliative care: Health care that aims to improve the quality of life of people facing life-limiting illnesses, through pain and symptom relief, and through psychosocial support for patients and their families. Palliative care can be delivered in tandem with curative treatment but its purpose is to care, not to cure.
Life-limiting illness: A broad range of conditions in which painful or distressing symptoms occur; although there may also be periods of healthy activity, there is usually at least a possibility of premature death.
Hospice: A specialist medical facility that provides palliative care. Hospices can be residential or outpatient facilities.
Chronic pain: As used in this report, pain that occurs over weeks, months, or years, rather than a few hours or a few days. Because of its duration, moderate to severe chronic pain should be treated with oral opioids rather than repeated injections, especially for children and people who are emaciated by diseases such as cancer and HIV/AIDS.
Opioid: Drugs derived from the opium poppy and similar synthetic drugs. All strong pain medicines, including morphine and pethidine, are opioids. Weaker opioids include codeine and tramadol.
Morphine: A strong opioid medicine, the gold standard for treatment of moderate to severe pain. Morphine is considered an essential medicine by the World Health Organization in its injectable, tablet, and oral solution formulations. Oral solution mixed from morphine powder is the cheapest formulation.
Basic pain medicines: Non-opioid pain medicines suitable for mild pain. These include paracetamol (also known as acetaminophen), aspirin, diclofenac, and ibuprofen.
Opioid dependence: Physical dependence experienced by a patient treated with opioids over time, such that withdrawal symptoms occur if the opioid is stopped abruptly. Physical dependence is treated by gradually reducing the opioid dose. It is distinct from addiction, a pattern of behaviors including compulsive use of drugs despite harm, which is uncommon in patients receiving opioid pain treatment.
Primary healthcare facility: A medical facility that a patient will usually attend first in a non-emergency situation, such as a clinic or healthcare center. Many patients globally only have access to primary-level health care.
Tertiary hospital: A large hospital at the peak of a hierarchy of hospitals. A tertiary hospital provides all of the major medical services available in a country and admits patients referred from smaller hospitals that provide fewer services.
Summary
Every year, tens of millions of people around the world with life-threatening illnesses suffer unnecessarily from severe pain and other debilitating symptoms because they lack access to palliative care, an inexpensive health service that aims to improve the quality of life of people with serious health conditions. As Human Rights Watch has documented, their suffering is often so intense they would rather die than live with their pain.
Although the World Health Organization (WHO) considers palliative care an integral component of cancer care and has urged countries to improve its availability, too often palliative care continues to be the neglected child of the health care family, receiving low priority from health policy makers and health care professionals and almost no funding. This is despite the fact that experts estimate that 60 percent of those who die each year in the developing world—a staggering 33 million people—need palliative care.[1] In part, this is because most cancer patients in developing countries are diagnosed when they have advanced disease and cannot be cured, so the only treatment option is palliative care.
Fifty years ago this year, the world community adopted the 1961 Single Convention on Narcotic Drugs, which stated that narcotic drugs are “indispensible for the relief of pain and suffering,” a core function of palliative care. It also instructed states to make adequate provision to ensure their availability. Yet, today these essential pain relieving drugs continue to be so poorly available in most of the world that WHO estimates that each year tens of millions of people suffer untreated moderate to severe pain, including 5.5 million terminal cancer patients and 1 million patients in the last phases of HIV/AIDS.[2]
In 2009 and 2010 Human Rights Watch surveyed palliative care experts in 40 countries to map the barriers that impede the availability of palliative care and pain treatment worldwide. We asked them about the situation in their country in three areas that WHO has said are critical to the development of palliative care: health policy, education of healthcare workers, and drug availability. We also analyzed publicly available data from all countries on consumption of opioid medications that can be used to treat chronic pain and compared them to cancer and AIDS mortality data to assess how well the need for pain treatment is met.
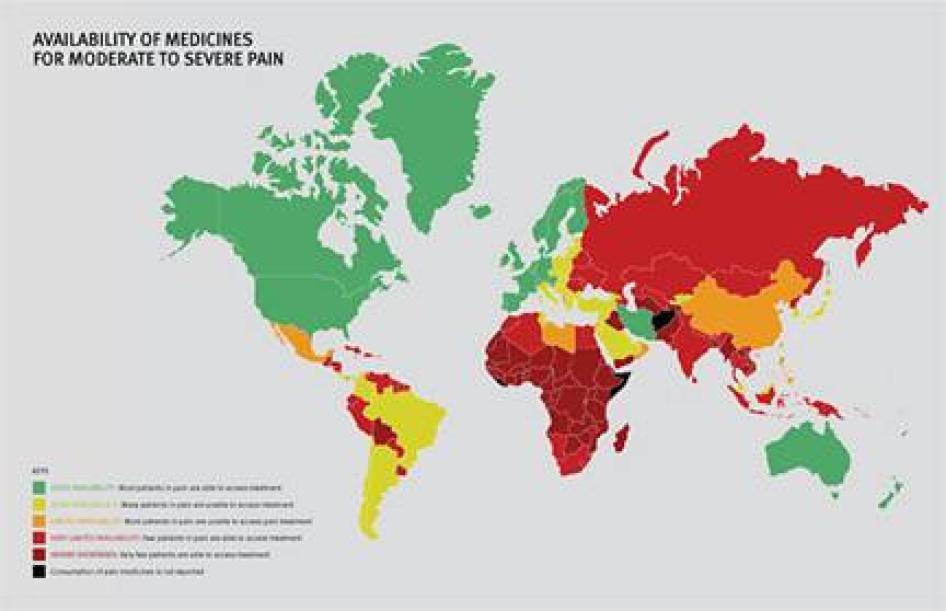
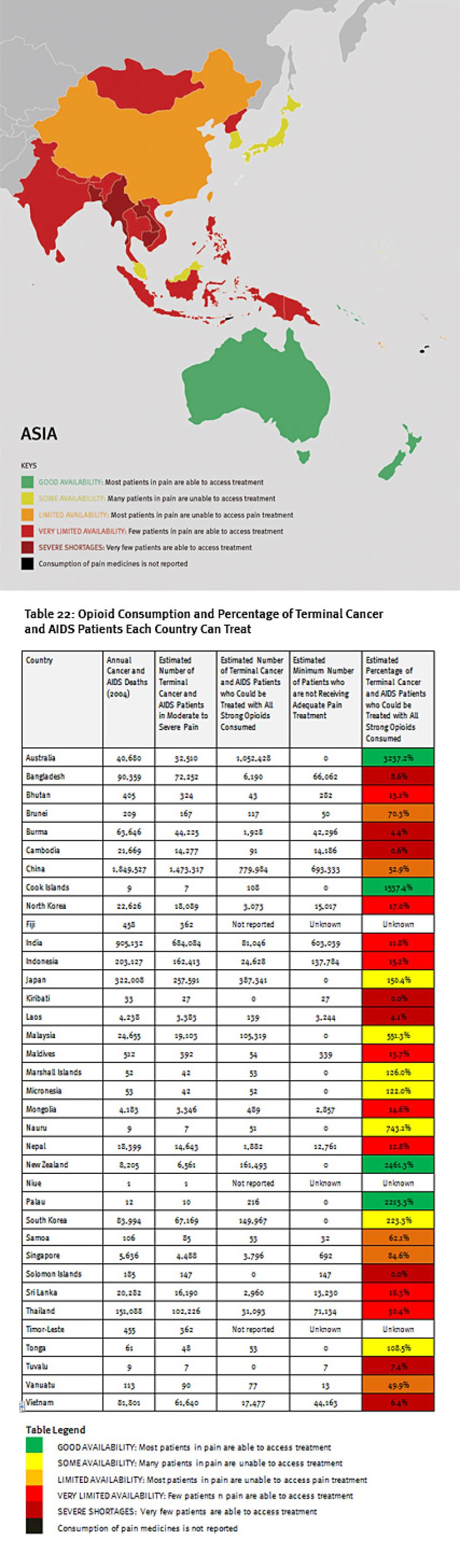
We found enormous unmet need for pain treatment. Fourteen countries reported no consumption of opioid pain medicines between 2006 and 2008, meaning that there are no medicines to treat moderate to severe pain available through legitimate medical channels in those countries. In a further eight countries that do not report their consumption of opioids, the situation is likely similar, as governments participating in the international drug control regime will not export opioids to those that do not. Thirteen other countries do not consume enough opioids to treat even one percent of their terminal cancer and HIV/AIDS patients. These countries are concentrated in Sub-Saharan Africa, but are also found in Asia, the Middle East and North Africa, and Central America.
Some of the world’s most populous countries have very poor availability of opioids for pain relief. Consequently, in each of China, India, Indonesia, Nigeria, Russia, and South Africa, at least 100,000 people die from cancer or HIV/AIDS each year without access to adequate pain treatment. The combined suffering due to lack of opioid pain medicines worldwide is staggering. Our calculations confirm that more than 3.5 million terminal cancer and HIV/AIDS patients die each year without access to adequate pain treatment, a very conservative estimate that assumes that all opioids are used to treat this patient group. It should be considered merely an indicator of the enormous unmet need for pain treatment. In reality, the limited opioids that are available are used to treat patients suffering pain from other causes too. So the real number of terminal cancer and HIV/AIDS patients with untreated pain must be higher, and many other patients with non-terminal cancer, HIV/AIDS, and with other diseases are also suffering untreated pain.
In many of the 40 countries surveyed we found multiple barriers to palliative care in each of the three areas. Only 11 of the countries surveyed have a national palliative care policy, despite WHO’s recommendation that countries put in place such policies. Most of the countries surveyed have inadequate opportunities for medical education in pain management or palliative care and in four of the countries surveyed–Cameroon, Ethiopia, Jordan, and Tanzania–no such education is available at all.
Thirty-three of the forty countries surveyed impose some kind of restrictive regulation on morphine prescribing that is not required by the international drug conventions. Thirty-one of the countries require that a special prescription form be used to prescribe morphine, and fourteen require doctors to have a special license to prescribe morphine. Although WHO has recommended that countries consider allowing nurses to prescribe morphine in order to improve accessibility to this essential medicine, only three countries (the United States and Uganda) do so.[3]
Our survey also identified some bright spots. Countries like Colombia, Jordan, Romania, Uganda, and Vietnam have undertaken comprehensive reform programs to improve access to palliative care. In these countries, leaders from the medical community have worked with domestic and international NGOs and their own governments to assess what barriers are preventing patients from accessing pain treatment and to address these barriers through policy development, law reform, and improving medical education and drug supply.
Governments have an obligation to address the widespread and unnecessary suffering caused by the poor availability of palliative care worldwide. Under international human rights law, governments must ensure equal access to the right to health and take reasonable steps to protect all against inhuman and degrading treatment. This should mean that health policies address the needs of people who require palliative care services; that healthcare workers have at least basic palliative care knowledge and skills; that medications like morphine are available throughout the country; and that drug regulations do not impede the ability of patients facing severe pain to get appropriate treatment. Failure to take such steps will likely result in a violation of the right to health. In some cases, failure to ensure patients have access to treatment for severe pain will also result in violation of the prohibition of cruel, inhuman, and degrading treatment.
The international community should address the poor availability of palliative care with urgency. Although WHO has urged countries to ensure the availability of palliative care, its governing body, the World Health Assembly, has largely been silent on the issue, despite the large numbers of people who require palliative care and the great suffering lack of palliative care causes. It needs to urgently show leadership and instruct its members to take effective steps to improve palliative care.
In recent years, the UN drug policy bodies, the Commission on Narcotic Drugs (CND), the UN Office on Drugs and Crime (UNODC), and the International Narcotics Control Board (INCB), have significantly increased the amount of attention that they pay to the availability of strong pain medications. In 2010 CND adopted a resolution on the issue, and UNODC discussed it prominently in the World Drug Report, its flagship publication. In 2011 the INCB published a special supplement to its Annual Report devoted to this issue. Improving access to essential medicines should be one aspect of a greater emphasis on promoting human rights within the UN drug policy bodies’ work. To mark the 50th anniversary of the 1961 Single Convention on Narcotic Drugs, these UN bodies should build on this momentum and develop concrete plans to implement the CND resolution and the INCB’s recommendation.
I. Background: Palliative Care and Pain Treatment
The Need for Palliative Care and Pain Treatment
Palliative care is a compassionate response to the suffering of patients with life-limiting illnesses like cancer or HIV/AIDS. It seeks to improve the quality of life of patients and their families facing life-limiting illness. Unlike curative health care, its purpose is not to cure a patient or extend his or her life, but rather to prevent and relieve pain and other physical, psychosocial, and spiritual problems. As Dame Cicely Saunders, founder of the first modern hospice and a lifelong advocate for palliative care, is widely reported to have said, palliative care is about “adding life to the days, not days to the life.”
The World Health Organization recognizes palliative care to be an integral part of health care for cancer, HIV/AIDS, and various other health conditions, that should be available to those who need it.[4] While palliative care is often associated with cancer, a much wider circle of patients with health conditions that limit their ability to live a normal life can benefit from it, including those with dementia, heart, liver or renal disease, or chronic and debilitating injuries. Palliative care is often provided alongside curative care services.
WHO has emphasized that palliative care is particularly important in developing countries, where the burden of HIV/AIDS is greatest, treatment is not universally available, and many patients with cancer seek medical attention only when the disease is in an advanced stage, beyond cure but causing severe pain.[5]While palliative care providers may offer inpatient services at hospices or hospitals, their focus is frequently on home-based care for people who are terminally ill or have life-limiting conditions, thus reaching people who otherwise might not have any access to healthcare services, including pain management. WHO has urged countries with limited resources to focus on developing home-based palliative care services, which can be provided by a visiting nurse or community health worker under the supervision of a doctor, making them very cost-effective.[6]
Moderate to severe pain is a common symptom of cancer and HIV/AIDS, as well as of many other health conditions.[7] A recent review of pain studies in cancer patients found that more than 50 percent experience pain,[8] and research consistently finds that 60 to 90 percent of patients with advanced cancer experience moderate to severe pain.[9]
Although no population-based studies of AIDS-related pain have been published, multiple studies report that 60 to 80 percent of patients in the last phases of illness experience significant pain.[10] Increasing availability of antiretroviral treatment (ART) in middle and low-income countries is prolonging the lives of many people with HIV. While people receiving ART generally have less pain than people who are not able to obtain it, many continue to experience pain symptoms.[11] ART can itself be a cause of pain, especially neuropathic pain caused by damaged nerves.[12]
The Consequences of Untreated Pain and Lack of Palliative Care
Moderate to severe pain, as well as other physical and psychosocial symptoms, have a profound impact on quality of life. Pain can lead to reduced mobility and consequent loss of strength; compromise the immune system; and interfere with a person’s ability to eat, concentrate, sleep, or interact with others.[13] A WHO study found that people who live with chronic pain are four times more likely to suffer from depression or anxiety.[14] The physical and psychological effects of chronic pain can directly influence the course of disease and also reduce patients’ adherence to treatment.[15]
Pain also has social consequences for patients and their caregivers, including inability to work, care for children or other family members, and participate in social activities.[16] At the end of life, pain can interfere with a patient’s ability to bid farewell to loved ones and make final arrangements.
Impact of Palliative Care and Pain Management
Most suffering caused by pain is avoidable as medicines to treat pain are effective, safe, inexpensive, and generally easy to administer.[17]WHO’s Pain Relief Ladder recommends the use of increasingly potent painkillers as pain becomes more severe, from basic pain medicines (such as acetaminophen, aspirin, or ibuprofen) to strong pain medicines such as morphine.[18]
Like morphine, all strong painkillers are opioids: extracts of the poppy plant or similar synthetic drugs. WHO’s Model List of Essential Medicines includes morphine in oral tablet, oral solution, and injectable formulations.[19] For chronic pain management, WHO recommends oral morphine given at regular intervals around the clock.[20]Patients can easily take oral morphine in their own homes and prescribing it avoids the pain of regular injections, which is especially important for children and patients whose muscle tissue is emaciated by cancer or HIV/AIDS. Similarly, with relatively inexpensive interventions, palliative care providers can treat a variety of other symptoms that are common among people with life-threatening illnesses, including breathlessness, nausea, anxiety, and depression.
Numerous studies have shown that patients who receive palliative care enjoy greater quality of life, have fewer distressing physical symptoms, and a lower incidence of depression or anxiety. A recent study published in the New England Journal of Medicine found that, in addition to improving quality of life, when palliative care was started shortly after diagnosis in patients with metastatic lung cancer, they actually lived an average of three months longer than patients that did not have access to palliative care.[21]
The Palliative Care and Pain Treatment Gap
WHO and the INCB have repeatedly drawn attention to the enormous unmet need for pain treatment and called for countries to meet this need through low-cost palliative care services. WHO estimates that tens of millions of people each year suffer untreated moderate to severe pain, including 5.5 million terminal cancer patients and 1 million patients in the last phases of HIV/AIDS. The president of the INCB has stated that access to morphine and other strong pain medicines is “virtually non-existent in over 150 countries.”[22]
In 2006 the International Observatory on End of Life Care published a study that found that no palliative care activity could be identified in 78 of 234 countries reviewed; in 41 countries it found some preparation for palliative care delivery but no actual services; and in 80 countries it found “localized provision” of palliative care by a small number of isolated services. In only 35 countries did the study find that palliative care was “approaching integration” into health services.[23]
Barriers to Palliative Care and Pain Treatment
There is no lack of information about the reasons why so many people who suffer from life-limiting illnesses do not have access to adequate pain treatment and palliative care. In dozens of publications spanning several decades, WHO, INCB, health care providers, academics and others have chronicled the barriers in great detail.[24] A common theme of many of these publications is the failure of many governments around the world to take reasonable steps to improve access to pain treatment and palliative care services.
Barriers can be divided into three areas: lack of health policies in support of palliative care development; lack of relevant training for healthcare workers; and poor availability of essential palliative care drugs. Within this latter category, there are a number of different common barriers, including the failure of states to put in place functioning drug supply systems, existence of unnecessarily restrictive drug control regulations and practices, fear among healthcare workers of legal sanctions for legitimate prescribing of opioid medications, and the unnecessarily high cost of pain medications. A more detailed discussion of these barriers can be found in Human Rights Watch’s March 2009 report, “Please do not make us suffer anymore…”: Access to Pain Treatment as a Human Right.
II. Survey Findings: Global Overview of Barriers to Pain Treatment
Our survey mapped barriers to palliative care related to health policy, education of healthcare workers, and drug availability in 40 countries. We asked healthcare workers questions about a number of common barriers in each of these areas to understand how widespread they are. The questions are based on research that Human Rights Watch previously conducted for its March 2009 report, “Please, do not make us suffer anymore…”: Access to Pain Treatment as a Human Right.[25]
The results of this survey confirm the general findings in that reportbut provide a more detailed picture of the specific barriers that exist in individual countries, as well as the prevalence of these barriers internationally. They provide a roadmap for individual countries and the international community for steps they need to take to improve palliative care availability. Our comparisons of consumption of opioid medications with mortality figures for cancer and HIV/AIDS demonstrate just how poor the availability of pain treatment is in many countries around the world.
We found enormous unmet need for pain treatment. Fourteen countries—Antigua and Barbuda, Bolivia, Cameroon, Comoros, Djibouti, Gambia, Guinea, Guinea-Bissau, Kiribati, Honduras, Swaziland, Solomon Islands, Tanzania and Tuvalu—reported no consumption of opioid pain medicines between 2006 and 2008, meaning that there are no medicines to treat moderate to severe pain available through legitimate medical channels in those countries.
In a further eight countries that do not report opioid consumption to the International Narcotics Control Board—Afghanistan, Belize, Equatorial Guinea, Fiji, Liberia, Niue, Somalia, and Timor-Leste—the situation is likely similar, as countries that participate in the international drug control regime undertake not to export opioids to these countries. Thirteen other countries—Burkina Faso, Burundi, Cambodia, Central African Republic, Chad, Cote d’Ivoire, Ethiopia, Haiti, Malawi, Mali, Niger, Nigeria, and Rwanda—do not consume enough opioids to treat even one percent of their terminal cancer and HIV/AIDS patients.
Of course, this means that in all of these countries, each year tens of thousands of patients suffer unnecessary pain. For example in Nigeria, more than 173,000 people with terminal cancer and HIV/AIDS patients need treatment for moderate to severe pain each year, but all the opioids consumed in Nigeria could treat just 274 such patients. In Ethiopia, more than 85,000 such patients need treatment, but there are drugs for less than 500. Less populous Cambodia still has more than 14,000 terminal cancer and HIV/AIDS patients suffering pain each year but drugs to treat just 91 of them. In addition to Nigeria, China, India, Indonesia, and Russia all have poor availability of opioids for pain relief and more than 100,000 patients who die from cancer or HIV/AIDS each year without access to adequate pain treatment.
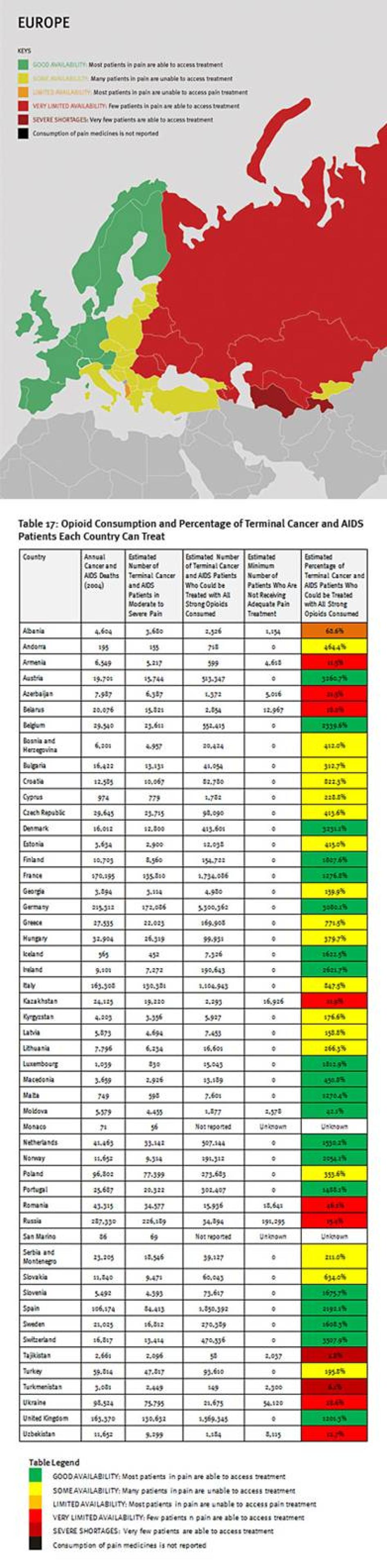
The combined suffering due to lack of opioid pain medicines worldwide is staggering. Our calculations confirm that more than 3.5 million terminal cancer and HIV/AIDS patients die each year without access to adequate pain treatment. This includes at least 1.7 million terminal cancer and HIV/AIDS patients in Asia, 1.2 million in sub-Saharan Africa, 480,000 in Europe, 180,000 in the Middle East and North Africa, and 100,000 in the Americas. It must be emphasized that these are very conservative estimates, which assume that all opioids are used to treat this patient group. This is why it is lower than WHO’s estimate that each year 5.5 million terminal cancer patients and 1 million patients in the last phases of HIV/AIDS suffer without pain treatment.[26]
Our calculations focus on patients with terminal cancer and HIV/AIDS because their need is great and because mortality data is available for these causes for most countries but not for many other diseases that cause immense pain. In reality, the limited opioids that are available are used to treat patients suffering pain from other causes, so the real number of terminal cancer and HIV/AIDS patients with untreated pain must be higher, and many other patients with non-terminal cancer, HIV/AIDS, and with other diseases are also suffering untreated pain. Consequently, the unmet need of terminal cancer and HIV/AIDS patients must be considered merely an indicator of even greater unmet need for pain treatment.
Availability of Policies that Promote Palliative Care and Pain Treatment
WHO has stressed the importance of comprehensive strategies to improve access to palliative care.[27] Without such policies, it is difficult to ensure that all relevant government and nongovernment agencies act in a coordinated fashion to address all barriers that impede the development of palliative care simultaneously. Under the right to health, countries are obliged to develop health policies that address the needs of the entire population, including people facing life-threatening illnesses.[28]
In our survey, we sought information about the availability of national palliative care policies; whether palliative care was addressed in national cancer and HIV control policies or plans; and whether oral and injectable morphine were included on national essential medicines lists.
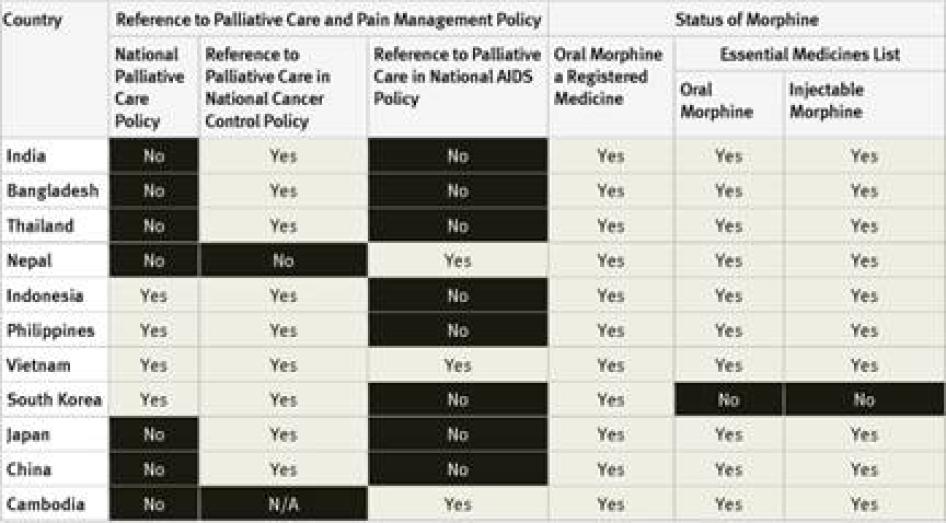
National Palliative Care Policies: Of the 40 countries surveyed, 29 did not have a national palliative care policy. Those that did are Argentina, Brazil, Indonesia, France, the Philippines, Poland, South Korea, Turkey, Uganda, the UK, and Vietnam. Although survey respondents were not directly asked about implementation, in two of these countries, Argentina and Brazil, the respondents told Human Rights Watch that the governments were not actually implementing the palliative care policies.[29] In Indonesia, survey respondents said that policies were only partially implemented.[30]
National Cancer Control Policies and Plans: National cancer control policies and plans of 24 of the 40 countries surveyed make reference to pain management or palliative care. Eight countries do not have a national cancer control policy or plan at all. In some countries, like India, the reference to palliative care is essentially rhetorical as it is not backed up by an action plan, targets, or budget allocation.[31] It is not clear in how many of the other countries surveyed that is the case.
National HIV/AIDS Control Policies and Plans: In 23 countries surveyed, national AIDS control policies did not make reference to palliative care, including three high-burden countries—Cameroon, Ethiopia, and Kenya.[32] AIDS control policies in 11 countries surveyed made reference to palliative care, including a number of high burden countries like South Africa, Tanzania, Nigeria, and Uganda. Four of the countries surveyed do not have a national AIDS control policy at all.
The fact that palliative care was mentioned in more than twice as many cancer policies may reflect the fact that palliative care has long been associated with cancer control. For example, WHO has made extensive recommendations on developing palliative care as part of cancer control programs but has saidlittle about its importance for patients with other diseases.[33] Palliative care and pain treatment have often been neglected in national and international responses to HIV/AIDS, despite significant prevalence of pain and other symptoms in people living with HIV/AIDS.[34]
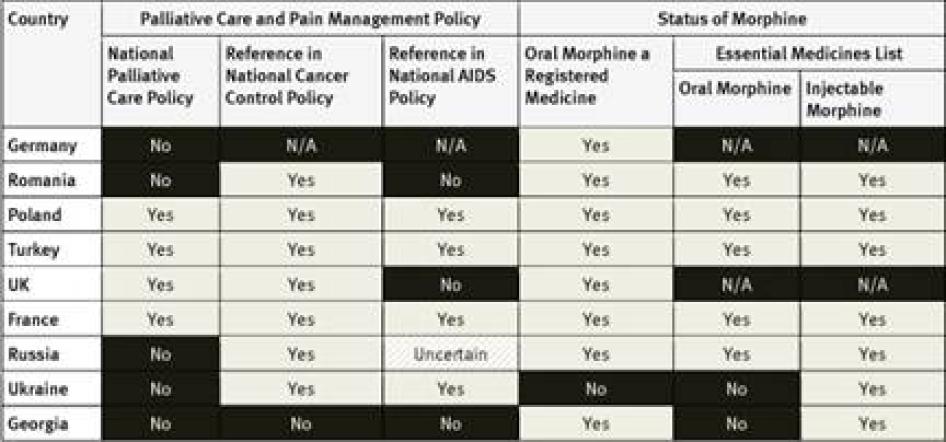
National Essential Medicines Lists: WHO considers injectable and oral morphine essential medicines for the treatment of pain that should be available to all people who need them.[35] Of the countries surveyed, only South Korea did not have injectable morphine on its essential medicines list; six had not included oral morphine: South Korea, Tanzania, Egypt, Iran, Ukraine and Georgia. Three countries–Germany, the United Kingdom (UK), and the United States (US)—do not have an essential medicines list.
Training for Healthcare Workers
One of the largest obstacles to the provision of good palliative care and pain treatment services in many countries is the lack of training for healthcare workers. Many do not have an adequate understanding of palliative care, do not know how to provide it and subscribe to various myths about morphine and other opioid analgesics. Key informants from 16 countries surveyed told us when asked whether healthcare workers feared potential legal repercussions when using opioid medications that the bigger problem was that healthcare workers in their countries were reluctant to use opioid medications because of exaggerated fears that they would cause dependence syndrome or respiratory distress in patients.[36]
To overcome these obstacles, WHO has recommended that countries provide training on palliative care to healthcare workers.[37] Under the right to health, countries are obliged to ensure that healthcare workers at least receive training in the basics of palliative care.[38] Given that almost all doctors will encounter patients in need of palliative care and pain treatment, instruction in these disciplines should be a standard part of undergraduate medical curriculum and postgraduate training in medical disciplines that routinely deal with patients who require palliative care.
In our survey, we sought information on the availability of instruction on palliative care in undergraduate and postgraduate medical studies as well as continuing medical education. We also asked key informants whether palliative care instruction in undergraduate studies was mandatory.
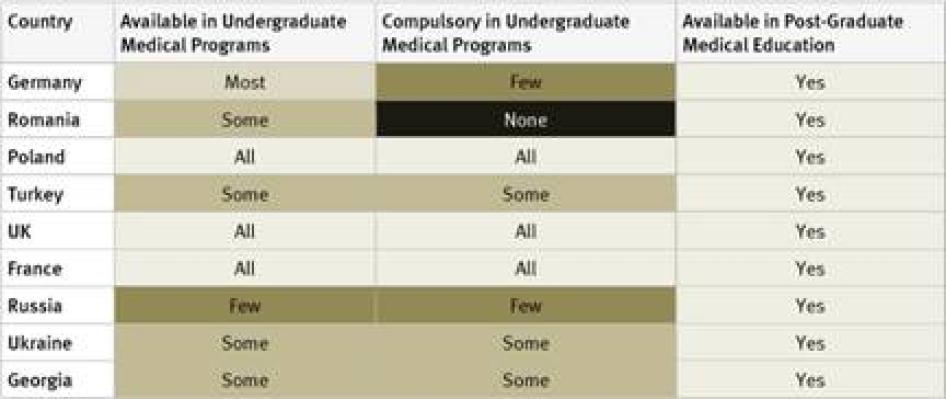
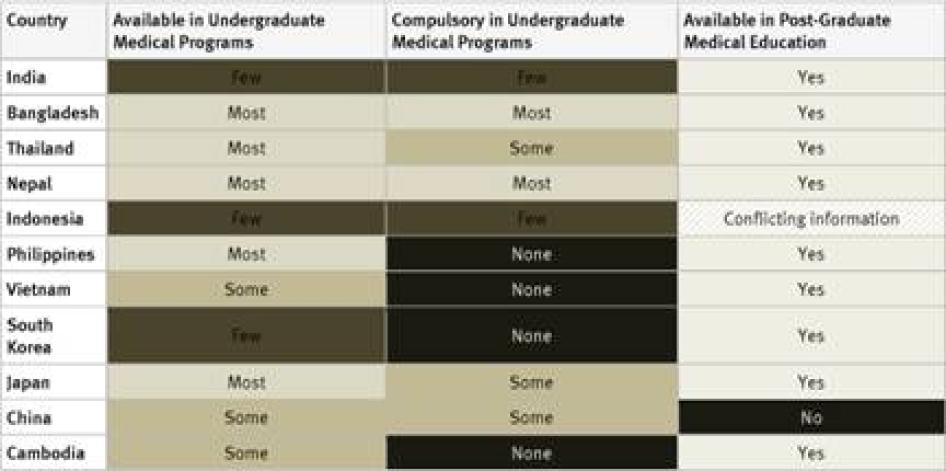
Undergraduate Medical Studies: Instruction in pain management (whether or not as part of instruction in palliative care) was available in all undergraduate programs in just five countries surveyed (France, Kenya, Poland, Uganda, and the United Kingdom). It was compulsory for undergraduate medical students in four of them: France, Poland, Uganda, and the United Kingdom. In Germany, compulsory instruction in palliative care in undergraduate medical studies will gradually be introduced starting in 2014.[39] In 33 of 40 countries instruction in pain management is available in some undergraduate medical programs.
Postgraduate Medical Studies: In the majority of surveyed countries—31 of 40—survey respondents reported that there are opportunities for postgraduate training in pain management (either as part of palliative care instruction or separately). In Ethiopia, Tanzania, Cameroon, Guatemala, Iran, Jordan, and China there is no postgraduate training in palliative care available at all. Many respondents, particularly in Africa and Asia, stated that healthcare workers who wanted to specialize in palliative care completed postgraduate training by correspondence or in foreign countries.
Drug Availability
Because of their potential for abuse, morphine and all other strong pain medicines are regulated under the Single Convention on Narcotic Drugs and national drug-control laws and regulations.[40] This means that their manufacture, import and export, distribution, prescription, and dispensation can only occur with government authorization, overseen by a body created by the Single Convention, the International Narcotics Control Board.
The fact that morphine and other strong analgesics are controlled medications has given rise to a host of problems related to their availability, as countries have struggled to put in place functioning supply and distribution systems; their accessibility, as many countries have enacted drug control laws that make it difficult for doctors to prescribe the medications and for patients to receive them; and their cost, as control measures and other factors have unnecessarily driven up the price of these medications, which can be produced at very low cost.
WHO has urged countries to put in place functioning supply and distribution systems and to ensure that drug control measures do not unnecessarily impede their availability and accessibility.[41] Under the UN drug conventions, countries are obliged to ensure the “adequate provision” of controlled medications while preventing their misuse or diversion.[42] Under international human rights law, countries are obliged to ensure the availability and accessibility of essential medications like morphine.[43]
In our survey, we sought information to assess the quality of countries’ supply and distribution systems for opioid analgesics, their drug regulations, and the cost of opioid analgesics.
Supply and Distribution System for Opioid Analgesics
As the import, production, and distribution of controlled medicines are under exclusive government control, they will simply not be available without government action to put in place effective supply systems. Governments need to provide annual estimates to the INCB for the amounts of morphine and other opioid medications needed. They must also approve production or import of such medications; provide licenses to health care providers and pharmacies before these can stock and dispense them; and authorize movements between producers, pharmacies, and health facilities.
In our survey, we sought to establish how widely available morphine is in different types of healthcare facilities in countries as a way of measuring the effectiveness of the supply and distribution systems governments have put in place. In particular, we asked about the availability of injectable morphine in hospitals and oral morphine in tertiary hospitals, other hospitals, pharmacies, health centers, hospices, and AIDS clinics. We also asked whether, in the experience of the key informants, morphine was harder to access outside major cities and whether health care providers were involved in developing their government’s estimates of its need for opioid medications.
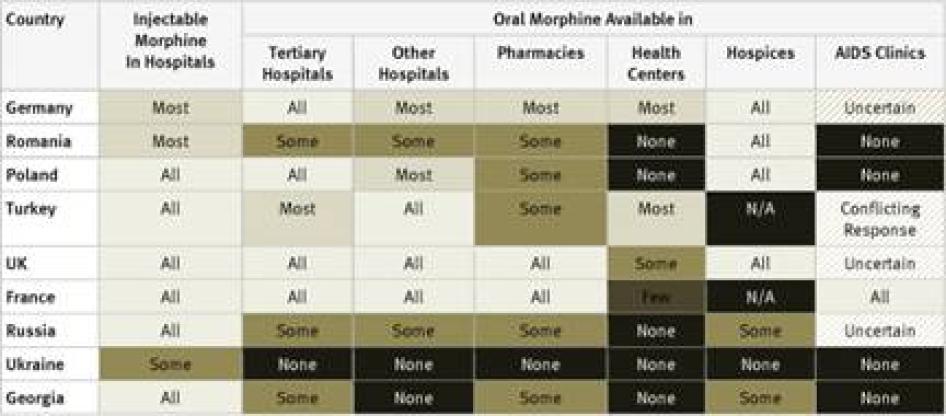
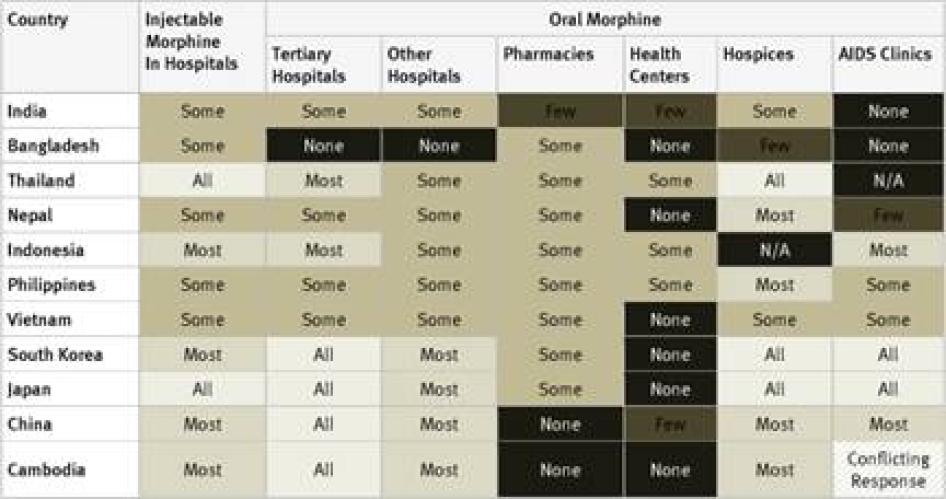
Injectable Morphine: Key informants reported that injectable morphine is available in all hospitals in just 10 of the 40 countries surveyed: France, Georgia, Iran, Japan, Poland, Russia, Thailand, Turkey, the UK, and the US. In a further 12 countries, it was reported to be available in most hospitals. Key informants said that injectable morphine was available only in “some” hospitals in the remaining 18 countries.
Oral Morphine: Two countries surveyed, Ukraine and Iran, do not have oral morphine at all. In Ukraine, despite recommendations by WHO to the contrary, injectable morphine is used to treat chronic pain, while in Iran a weaker oral opioid, Tramadol, is used. Table 1 contains an overview of the data on availability of oral morphine in the various healthcare settings.
Table 1: Availability of Oral Morphine in Different Healthcare Settings in Countries Surveyed
Health facility |
None |
Few |
Some |
Most |
All |
Don’t know |
N/A |
Tertiary hospitals |
3 |
- |
19 |
6 |
12 |
- |
- |
Other hospitals |
6 |
3 |
14 |
9 |
4 |
4 |
- |
Pharmacies |
6 |
5 |
24 |
3 |
2 |
- |
- |
Health centers |
22 |
5 |
10 |
3 |
- |
- |
- |
Hospices |
5 |
1 |
8 |
7 |
13 |
- |
6 |
AIDS clinics |
17 |
1 |
7 |
3 |
3 |
5 |
4 |
The table demonstrates that oral morphine is generally most widely available in tertiary hospitals and hospices; somewhat less available in pharmacies and smaller hospitals; and least likely to be available in health centers or AIDS clinics. In other words, patients with pain often need to be referred to larger health facilities, making pain treatment less accessible and more costly for them.
Poor availability of oral morphine in smaller healthcare facilities also compounds access problems for people who live far from major cities, where larger health facilities are likely to be located. In many developing countries, distance and the cost of travel make it very difficult for people living in rural areas to reach any health facility, but their closest facility is likely to be a small clinic or health centre or perhaps a pharmacy or small hospital. As oral morphine and other opioids are less likely to be available in these settings than in larger hospitals, rural patients’ barriers to accessing pain treatments are compounded.[44]
A key component of a functioning supply and distribution system is a robust process to estimate the need for opioid medications. WHO has recommended that the government involve healthcare workers in developing such estimates.[45] In 23 of the countries surveyed, healthcare workers, who were mostly leading palliative care or pain management specialists, were not aware of any such consultations.[46] In several other countries, survey respondents reported occasional consultations that were thought to be inadequate or have no real affect on the estimates process.[47]
Unsurprisingly, industrialized countries like Germany, France, the United Kingdom, and the United States had widespread availability of oral morphine across these settings. Besides Iran and Ukraine, which have no oral morphine, other countries that stood out as having particularly poor accessibility across the various health settings were scattered throughout the regions and included Bangladesh, Cameroon, Ethiopia, Georgia, Guatemala, Morocco, and Pakistan.
Drug Regulations
The Single Convention on Narcotic Drugs lays out three minimum criteria that countries must observe when developing national regulations governing the handling of opioids. First, individuals must be authorized to dispense opioids by their professional license to practice or be specially licensed to do so. Secondly, movement of opioids may only occur between institutions or individuals so authorized under national law. Finally, a medical prescription is required before opioids may be dispensed to a patient. Governments may, under the convention, impose additional requirements if deemed necessary.[48]But WHO has observed that the right to impose additional requirements “must be continually balanced against the responsibility to ensure opioid availability for medical purposes.”[49]
Many countries have adopted regulations that go well beyond the requirements of the Single Convention, often creating complex procedures for procurement, stocking, and dispensing of controlled medications that impede their accessibility for patients with a legitimate medical need. Under the UN drug conventions and international human rights law, countries must balance their efforts to prevent the misuse of controlled substances against the obligation to make them available to patients who need them.[50] Drug control regulations that have a disproportionately negative effect on availability and accessibility of controlled medications will violate both drug conventions and human rights treaties.
In our survey, we collected information about three types of regulations that are commonly reported to limit the accessibility of controlled medicines: special licensing requirements for healthcare workers; use of special prescription forms and other special prescription requirements; and limits on the amount of morphine that can be prescribed using one prescription or the length of time that a prescription can cover. We also asked key informants whether, in their experience, doctors were reluctant to prescribe opioid medications because of worries about potential legal scrutiny.
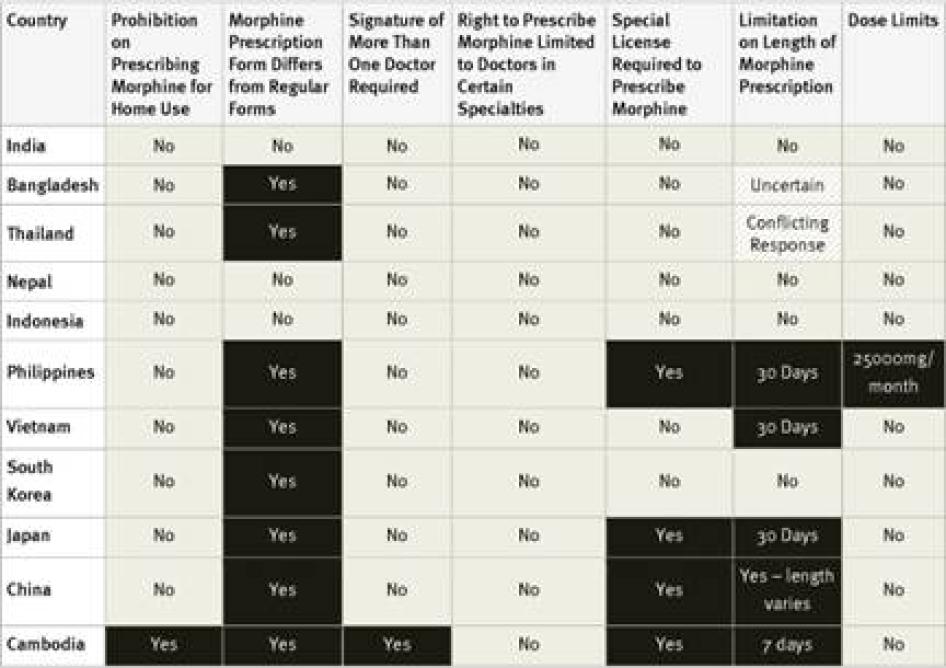
Special Licensing Requirements: The Single Convention on Narcotic Drugs requires that people who handle opioid medications be licensed to do so. The convention does not require a special license and in many countries healthcare workers are licensed to handle such medications by virtue of their professional license. Yet many countries require a special license and some allow only certain types of doctors to prescribe opioid medications. WHO has recommended that “physicians, nurses and pharmacists should be legally empowered to prescribe, dispense and administer opioids to patients in accordance with local needs.”[51]As patients who suffer pain have a right to access essential medicines including morphine, the right to the highest attainable standard of health requires that limits on which healthcare workers can prescribe opioids be no more restrictive than is reasonably necessary to prevent their diversion to misuse.[52]
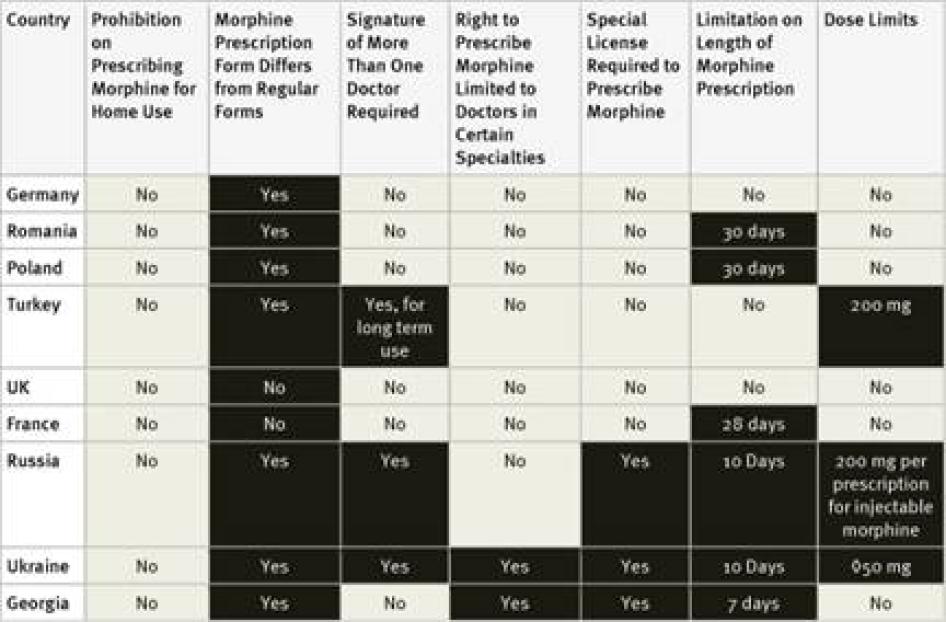
Fourteen of the forty countries surveyed require doctors to obtain a special license or registration in order to prescribe controlled medications. Survey respondents in some countries, such as the United States, said that the process for obtaining this special license is simple and almost all doctors have one.[53]Others said that obtaining the necessary license requires considerable paperwork or even invasive screening of the doctor. For example, the Philippines requires doctors applying for a license to submit urine for drug tests.[54]In Ukraine, doctors must obtain certificates from the police department and drug treatment clinic that they do not have a criminal record or are not registered as drug users. Survey respondents from Morocco and the Philippines stated that, as a result of complex licensing procedures, very few doctors have a license to prescribe opioids.[55]
Egypt, Ukraine, and Georgia limit the right to prescribe opioids to doctors practicing in certain specialties, commonly oncology, pain management, or anesthesiology.[56] In Russia, physicians who do not work in the government health care system cannot prescribe opioids.[57]
Only 2 of 40 countries, Uganda and the United Kingdom, allow nurses to prescribe controlled medicines in certain circumstances. In a third country, the United States, most but not all states allow nurses to prescribe. In South Africa, efforts to introduce nurse-prescribing are underway.[58] Nurse-prescribing is essential in resource-limited settings where doctor-patient ratios are very low and many people never see a doctor in their lifetime.[59] The INCB has commended Uganda for introducing nurse-prescribing.[60]
Special Prescription Requirements: The Single Convention does not require prescriptions for controlled medicines to be written on special prescription forms but does explicitly permit this practice. WHO has observed that special multiple-copy prescription requirements “typically reduce prescribing of covered drugs by 50 percent or more.”[61] While the use of special prescription forms and procedures is not by definition inconsistent with the right to health, they must be easily accessible for healthcare workers and not add cost to the medicines.
Our survey found that 30 of the 40 countries surveyed require special prescription forms. In two countries, Germany and Morocco, survey respondents mentioned that doctors have to apply to receive the forms; in the Philippines they have to pay for them.[62] Survey respondents in three countries, El Salvador, Turkey, and Ukraine, mentioned problems accessing enough special prescription forms.[63]
In three countries, Russia, Ukraine and—for longer prescriptions—Turkey, prescriptions for morphine must be approved by more than one doctor. In Ukraine, such prescriptions must be made by a group of three doctors, one of whom must be an oncologist, and approved by the chief doctor of the hospital.[64]
Prescription Limitations: WHO has recommended that “decisions concerning the type of drug to be used, the amount of the prescription and the duration of therapy are best made by medical professionals on the basis of the individual needs of each patient, not by regulation.”[65] Yet, many countries have regulations that unnecessarily constrict these medical decisions, in violation of patients’ right to the highest attainable standard of health.[66]
Our survey found that 25 of the 40 countries surveyed impose limits of these kinds. Some countries, including Ukraine and Turkey, limit the daily dose of morphine that can be prescribed and others, including Germany, Egypt, and Russia, limit the amount that can be prescribed in one prescription, and others, including the Philippines, set a maximum monthly dose. Other countries limit the number of days that a morphine prescription can cover. In our survey, the shortest daily limits were seven days in Cambodia, Egypt, Morocco, and Georgia, and ten days in Argentina, Russia, and Ukraine. Jordan imposes a limit of ten days for cancer patients and just three days for other patients. In China, the limit varies according to the morphine formulation, fifteen days for immediate release morphine tablets, seven days for slow release tablets, and just three days for injectable morphine.[67]
Fourteen of the countries surveyed do not impose a time limit on the number of days one prescription can cover: the United States, Germany, Turkey, the United Kingdom, Pakistan, Nigeria, Uganda, Tanzania, Nepal, India, Ethiopia, Indonesia, Kenya, and South Korea. Another 15 of the countries maintained a limit of 28 to 30 days.
Fears of Legal Sanction: Regulations that contain ambiguous standards regarding medical prescription and handling of opioids, or punish mishandling harshly, can chill legitimate prescribing. The INCB has said the “vast majority of health professionals exercise their activity within the law and should be able to do so without unnecessary fear of sanctions for unintended violations.”[68]Criminalizing unintentional mistakes in opioid prescription is not consistent with the right to health.[69] Countries must ensure that regulations are unambiguous and that complete information about them is readily available for health care providers, so that they do not unreasonably chill opioid prescribing, denying patients pain treatment.
Key informants from 34 of 40 countries said that doctors were hesitant to prescribe opioids because of fear of legal sanction for mishandling them, such as criminal sanctions or professional sanctions such as license revocation. Only key respondents from Thailand, France, Romania, Japan, Colombia, and Cameroon felt that healthcare workers have no fears of legal sanction sufficient to deter prescribing such medications.
Cost of Opioid Medications
Basic oral morphine in powder or tablet form is not protected by any patent and can be produced very cheaply. In India, basic morphine tablets are sold for as little as US$0.017 or about US$0.12 for a typical daily dose.[70]Yet, the actual cost of morphine is much higher in many countries due to a variety of factors that drive up the price, including government regulation, licensing and taxation, poor distribution systems, low demand, large overhead of local production, and price regulation by some industrialized governments.[71] In some countries, the promotion of non-generic and costly forms of opioid analgesics has resulted in pharmaceutical companies withdrawing inexpensive formulations.[72] Paradoxically, morphine is often more expensive in low- and middle-income countries than in industrialized countries.[73]
The International Association for Hospice and Palliative Care recommends that “no government should approve modified release morphine … without also guaranteeing widely available normal release oral morphine.”[74] Under the right to health, governments are obliged to ensure that both immediate release and slow release morphine tablets are available, as both are included in the WHO’s Model List of Essential Medicines.[75] They must also explore ways to ensure that morphine is available at the lowest cost and is affordable to all people who need it, including by taking steps to ensure that government regulation does not disproportionately affect cost and considering subsidies for poor patients.[76]
Few of the healthcare workers surveyed were able to provide comprehensive information about the cost of morphine in their countries, but various healthcare workers did discuss the following matters:
Availability of Expensive Formulations: Survey respondents in Bangladesh, Thailand, Ecuador, South Africa, and South Korea reported that inexpensive immediate release oral morphine was not available while more costly slow-release oral morphine tablets were.
Subsidies: Subsidies can help ensure the affordability of pain medications. Survey respondents in Colombia, Egypt, Russia, and Uganda reported that their governments provide at least one formulation of morphine free of charge to all patients. Respondents from 13 countries –France, Georgia, China, Germany, Japan, Kenya, Mexico, Poland, Romania, South Africa, South Korea, Thailand, and the United Kingdom—said governments offered at least partial subsidies in some circumstances. In some of these countries, morphine was subsidized only for patients with low incomes or for hospital inpatients but not for outpatients, an approach that is inconsistent with WHO’s recommendation that countries prioritize developing home-based palliative care.[77] In France, Georgia, Poland, and Romania, there are greater subsidies for cancer patients than other patients.This reflects the reality, discussed above, that WHO has made extensive recommendations on developing palliative care as part of cancer control programs but has said little about its importance for patients with other diseases.[78] This approach could violate governments’ obligations to uphold the right to the highest attainable standard of health and specifically to provide essential medicines without discrimination on the basis of health status.[79]
Best Practices: Addressing Barriers to Pain Treatment and Palliative Care through Comprehensive Reform
In most low and middle-income countries, an assessment of barriers to access to morphine and the development of a plan of action must be the first step in a comprehensive effort to address those barriers. To be successful, reforms must address both supply and demand for morphine simultaneously; improving supply chains to increase morphine stocks will not improve patient access unless doctors are also adequately trained in pain treatment and palliative care, and vice-versa. In undertaking these reforms, governments can draw upon the expertise of the INCB and WHO. There are several nongovernmental organizations (NGOs) that work to improve the availability of medicines in developing countries, including Supply Chain Management Systems, the IDA Foundation, and Health Action International.[80] On the whole, these NGOs have yet to turn their efforts to the availability of opioid pain medicines, but they have considerable relevant expertise that governments could draw upon.
A number of countries have begun comprehensive reform efforts aimed at improving access to pain treatment and palliative care, with support from international organizations and have had some initial success. Such efforts in Uganda, Vietnam, Jordan, Colombia, and Romania are profiled in this report.
Map of Sub-Saharan Africa
III. Sub-Saharan Africa
Regional Overview
"Before I came [to Kenyatta National Hospital], I couldn't eat or breathe well [because of the pain]. Now that I have been given medicine [morphine], I can eat and breathe. I couldn't sit down, but now I can. I had pain for more than a month. I told the doctor and nurses [at another hospital] that I had pain. It took too long to get pain treatment… Here I got it immediately and started feeling well again."
– Christine L., an 18 year-old woman with Breast Cancer, Nairobi, Kenya.
"We have no pethidine, no DF-118 (dihydrocodeine) and no morphine.... We have children here with advanced HIV; some are in severe pain. The pain management for children with advanced HIV is not enough."
– Nurse, Bondo District Hospital, Kenya.
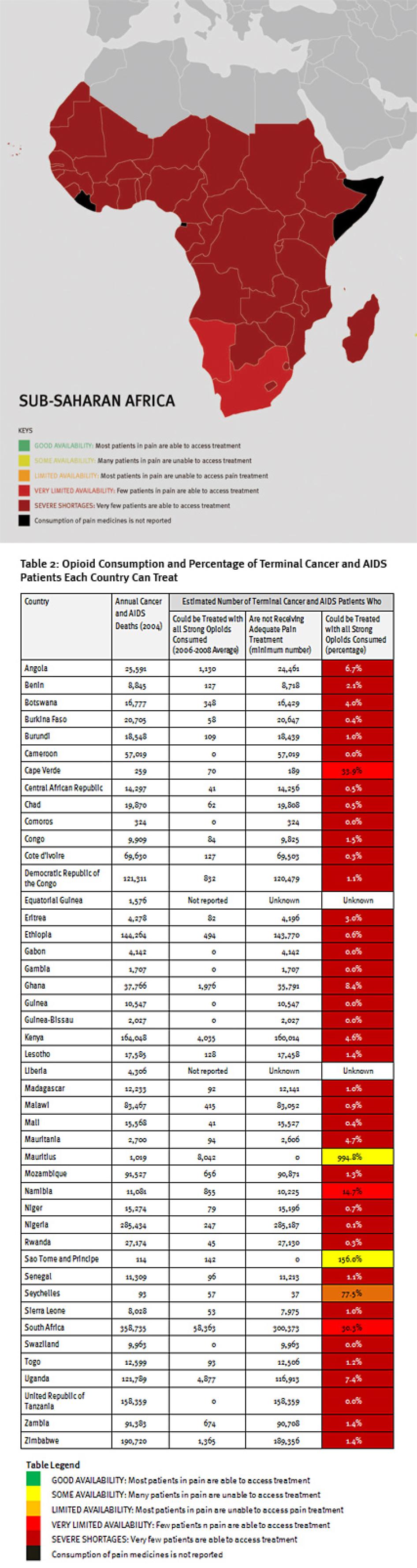
Sub-Saharan Africa has the lowest consumption of opioid analgesics worldwide. As shown in Table 2, 37 sub-Saharan African countries consume so few opioid medications that even if they were used exclusively to treat pain in patients with terminal cancer and HIV, fewer than 10 percent of those patients could receive adequate pain treatment. Eighteen countries could not treat even one percent of this group of patients, and eight countries reported no consumption of opioids at all during 2006 to 2008.
Healthcare workers who must treat patients in facilities with no pain medications understandably express frustration. When Human Rights Watch visited a Kenyan hospital that had no opioid pain medicines a nurse showed us a child who had suffered severe burns, and said: “If we had diclofenac [a weak non-opioid pain reliever] we would give it, but we don’t have it here.”[81]
Consequently, at least 1.2 million people in sub-Saharan Africa die from cancer or HIV/AIDS without adequate pain treatment each year. This is a very conservative estimate, which assumes that all opioids are used to treat this patient group. It should be considered merely an indicator of the enormous unmet need for pain treatment. In reality, the limited opioids that are available are used to treat patients suffering pain from other causes too. So the real number of terminal cancer and HIV/AIDS patients with untreated pain must be higher, and many other patients with non-terminal cancer and HIV/AIDS and with other diseases are also suffering untreated pain.
While the challenging economic environment and poor health care infrastructure undoubtedly are a major reason for this situation, our survey findings suggest that government failure to take reasonable, low-cost steps to improve availability of opioid analgesics is a significant contributing factor in many countries.
The African countries we surveyed have inadequate government policies to promote palliative care, inadequate medical education in pain treatment and palliative care, and very poor availability of morphine across different healthcare settings, indicating poor supply and distribution systems for opioids. African countries surveyed imposed few of the regulatory restrictions covered in this survey, but in all but one of the countries surveyed respondents reported that physicians’ fears about possible legal sanctions are a barrier to prescribing opioids.
The survey findings highlight that even poor countries can make significant progress in delivering palliative care. Concerted efforts by Uganda’s government and civil society to improve access to palliative care have resulted in the removal of many of the barriers discussed in this survey. During 2006 to 2008 Uganda could already treat a significantly higher portion of its terminal HIV/AIDS and cancer patients than neighboring Kenya (Uganda: 7.4 percent; Kenya: 4.6 percent), even though Kenya’s GDP is significantly higher than Uganda’s. The ongoing process of improving access to palliative care in Uganda, discussed below, means that Uganda’s consumption of opioids has likely increased subsequent to 2008, so that more patients care receive treatment.
Policy
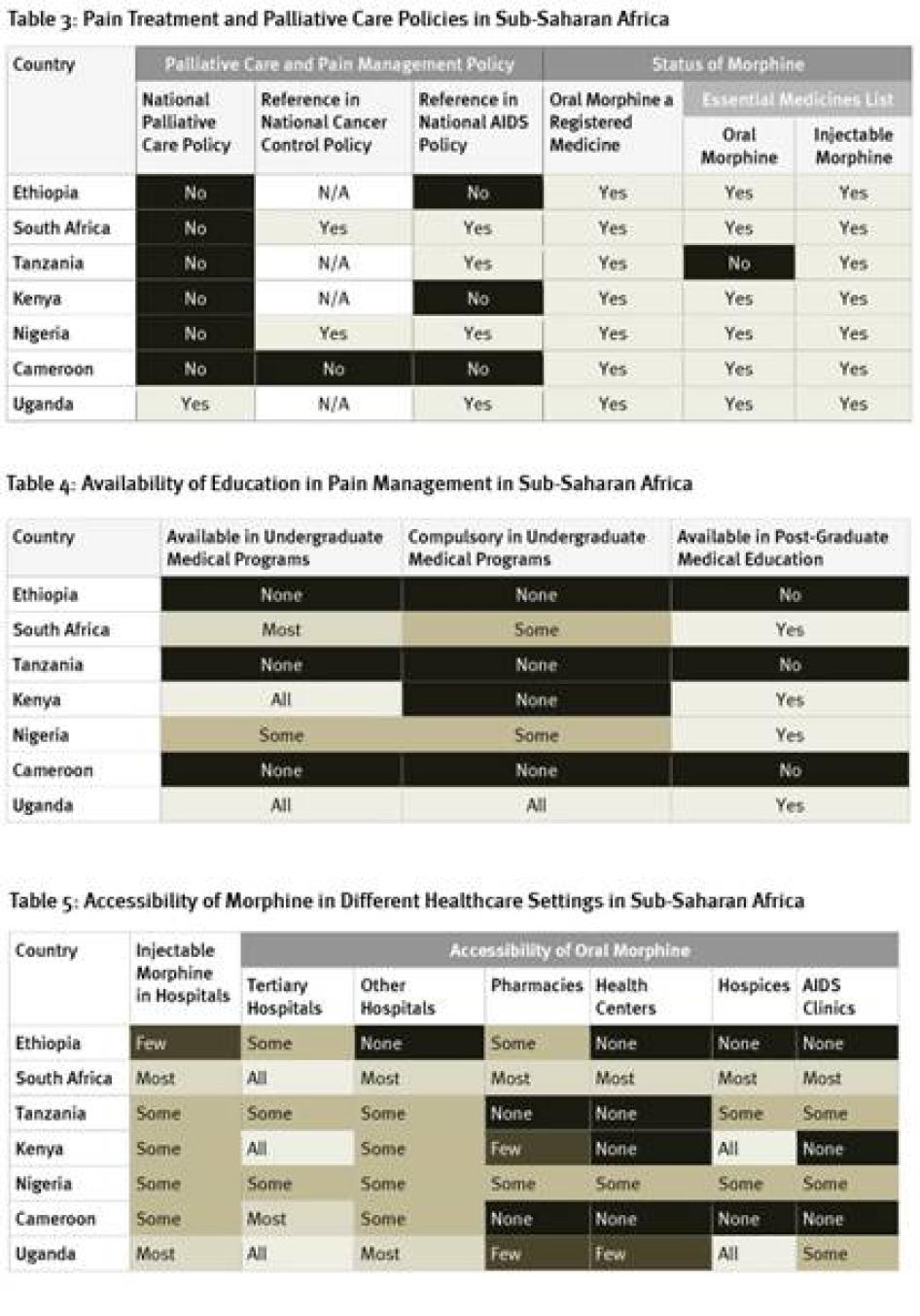
As Table 3 shows, there is little government support for palliative care in the seven African countries we surveyed, with Uganda the only country that has a national palliative care policy. Only two of the countries have cancer control policies that reference palliative care; four do not have such policies at all. Four countries surveyed had HIV policies that referred to palliative care but, despite high HIV/AIDS mortality levels, the HIV policies in three countries did not. More positively, most countries have included oral morphine in their essential medicines list although, as discussed below, it’s availability in practice is often very limited.
Survey respondents from Ethiopia and Kenya reported that those countries are currently developing cancer control strategies. Kenya’s strategy is expected to include palliative care.
Education
The availability of education in pain management varied greatly in the African countries surveyed. As Table 4 shows, in three of the seven countries surveyed—Cameroon, Ethiopia, and Tanzania—no instruction in pain management is available at all for physicians. On the other hand, in Uganda, palliative care instruction is compulsory in all undergraduate medical programs and available in postgraduate medical education.
Doctors from several of the African countries surveyed mentioned that some healthcare workers had received post-graduate education in palliative care from foreign institutions in other African countries and Europe through distance learning or programs run jointly between African and non-African institutions. This was the case in countries that had some domestic opportunities for post-graduate training, as well as those that did not. While these programs make an important contribution to building African expertise in palliative care, domestic programs are essential to adequately train sufficient numbers of healthcare workers in pain treatment and palliative care.
Drug Availability
Supply and Distribution
Weak supply and distribution systems are a key reason for the low consumption of morphine in Africa. As Table 5 shows, respondents in all seven countries surveyed said that injectable morphine was not available in all hospitals, although it is available in most hospitals in Uganda and South Africa. While oral morphine is available at all tertiary hospitals in Kenya, South Africa, and Uganda, it is only available in some tertiary hospitals in Ethiopia, Nigeria, and Tanzania. In Africa, most health care is provided in a primary healthcare setting such as a health care center or clinics. In four countries surveyed, no health centers have oral morphine. In two countries, even hospices do not have oral morphine. Overall, availability of morphine was best in South Africa and Uganda and worst in Ethiopia and Cameroon.[82]
While all countries surveyed have some availability of oral morphine, many other African countries do not. According to Anne Merriman, founder of Hospice Africa Uganda, several dozen countries in sub-Saharan Africa, including all 31 Francophone countries except Cameroon, do not have this essential medicine.[83]
Drug Regulations
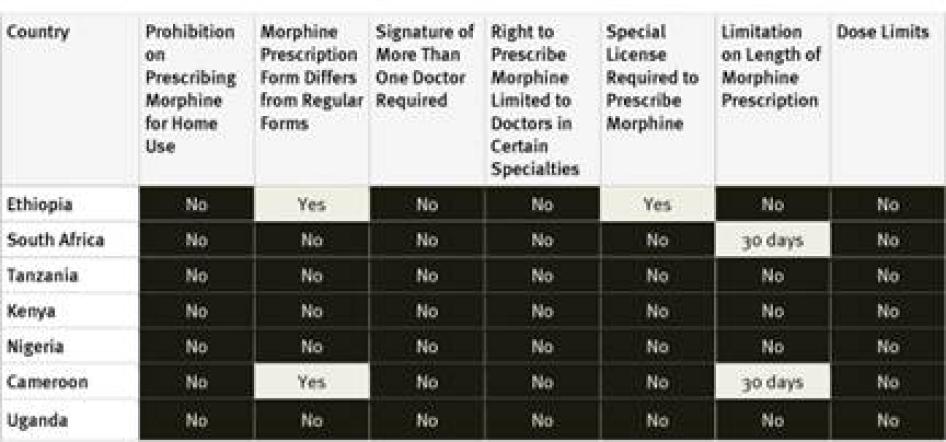
Our survey found relatively few restrictive drug regulations in African countries surveyed. None of the countries surveyed impose arbitrary dose limits on prescriptions or restrict prescribing rights to certain types of physicians. None of the countries require a special license for physicians to be allowed to prescribe opioid medications; although Ethiopian drug legislation in a UNODC database states a special license is required, survey respondents said the provision is not enforced in practice.[84] Only two of the seven countries, Ethiopia and Cameroon, require a special prescription form. Cameroon and South Africa cap the number of days a prescription for opioid medications can cover at 30 days; other countries surveyed did not impose any limit. Key informants from Tanzania reported that while the country’s regulations do not require a special prescription form or impose a limit on the time that prescriptions can cover, some individual medical institutions do impose these.
While our survey found few regulatory barriers, key informants from all African countries surveyed except for Cameroon reported that healthcare workers fear legal sanctions for prescribing opioid medications and identified this fear as a barrier to prescribing them.
Although Uganda is leading the world by developing a program to train nurses to prescribe opioids, most of the African countries surveyed still do not allow nurse-prescribing. Because of low numbers of doctors and large populations living great distances from the nearest doctor or unable to afford the transport to travel to a doctor that is relatively close, allowing trained nurses to prescribe morphine is essential for increasing access to opioids in Africa. At present, only Uganda allows specially trained nurses and clinical officers to prescribe morphine. South Africa is considering changing its regulations to allow nurses to prescribe. Human Rights Watch researchers have previously learned that nurses in some African countries give patients opioids when no doctor is available to do so, although this is contrary to the law.
Table 6: Restrictive Regulation of Morphine Prescribing in Sub-Saharan Africa
Cost
Most of the African countries surveyed use oral liquid mixed from morphine powder, which can be prepared for just a few cents per dose. Nonetheless, for many Africans who subsist on less than US$1 per day, the cost remains prohibitive. Healthcare workers reported that many hospices and hospitals subsidize the cost of morphine for all their patients or for their poorest patients. The Ugandan government’s comprehensive effort to improve access to palliative care has included providing morphine free-of-charge.
Doctors in Tanzania reported that weak supply chains make the real cost of providing morphine a burden upon health care services, because staff must travel long distances to collect oral morphine solution, incurring travel expenses and lost staff time. When they are available, other morphine formulations are often significantly more expensive than the lowest price at which they can be purchased internationally, probably due to low demand and weak supply chains.
Best Practice and Reform Efforts: Uganda
In the last 10 years, Uganda has led the African continent in efforts to improve access to palliative care, making significant progress on a number of fronts. The Ugandan government has worked with WHO and religious and nongovernmental organizations to systematically address barriers to access to palliative care.
In its five-year Strategic Health Plan for 2000-2005, Uganda became the first African country to state that palliative care was an essential clinical service for all citizens. Since then, the government has worked to improve the availability of narcotic medications. It added liquid morphine to its essential drug list and adopted a new set of Guidelines for Handling of Class A Drugs for health care practitioners, also a first in Africa. The Ministry of Health also started importing oral morphine powder and providing oral morphine solution to public health facilities at no cost. Since 2000 opioid consumption in morphine equivalence has increased four-fold from less than 0.2 mg per person to almost 0.8 mg per person in 2008.[85]
The government’s efforts have not been limited to improved drug provision. In 2004 Ugandan law was amended to allow nurses and clinical officers, once they have completed a nine-month palliative care course, to prescribe morphine.[86] More than 80 nurses and clinical officers have since graduated from Hospice Africa Uganda’s Clinical Palliative Care Course. In its 2004 report the INCB commended Uganda’s efforts to improve access to pain treatment, including reforming Uganda’s narcotics control laws so that specially trained nurses could prescribe morphine.[87]
In recent years, Uganda has significantly boosted its capacity for palliative care. There are now at least 50 facilities providing palliative care services, including morphine.[88] In order to reach more patients in need, community services for home-based palliative care have been greatly strengthened. The current strategic plan states that all hospitals and health centers should provide palliative care, that necessary medicines should be available, and that palliative care should be integrated into the curriculum of health training institutions. It also emphasizes the need to strengthen referral systems and community-based palliative care.[89]
Uganda’s significant progress demonstrates the potential for government leadership to rapidly scale up access to palliative care through reforming laws and regulations, increasing drug provision, and encouraging education in palliative care. Many of the Ugandan government’s reforms were carried out in cooperation with NGO representatives and WHO. Government, NGO, and WHO representatives met at a conference to discuss drug treatment availability in 1998, where they made commitments to taking specific measures to improve drug treatment availability. These plans and commitments have translated to many of the country’s achievements today.[90]
Despite progress, many challenges remain in ensuring access to palliative care throughout Uganda. Some of the nurses trained in palliative care are not using their training because morphine is not available where they work or because hospital administrators are not supporting their efforts, for example, by failing to assign them to care for patients with life-limiting disease. District health departments do not have defined palliative care budgets and inadequate distribution systems for morphine remain a problem.[91] There is an ongoing need to ensure the availability of oral morphine throughout Uganda; to keep it affordable; prevent stock-outs; and train all relevant healthcare workers.
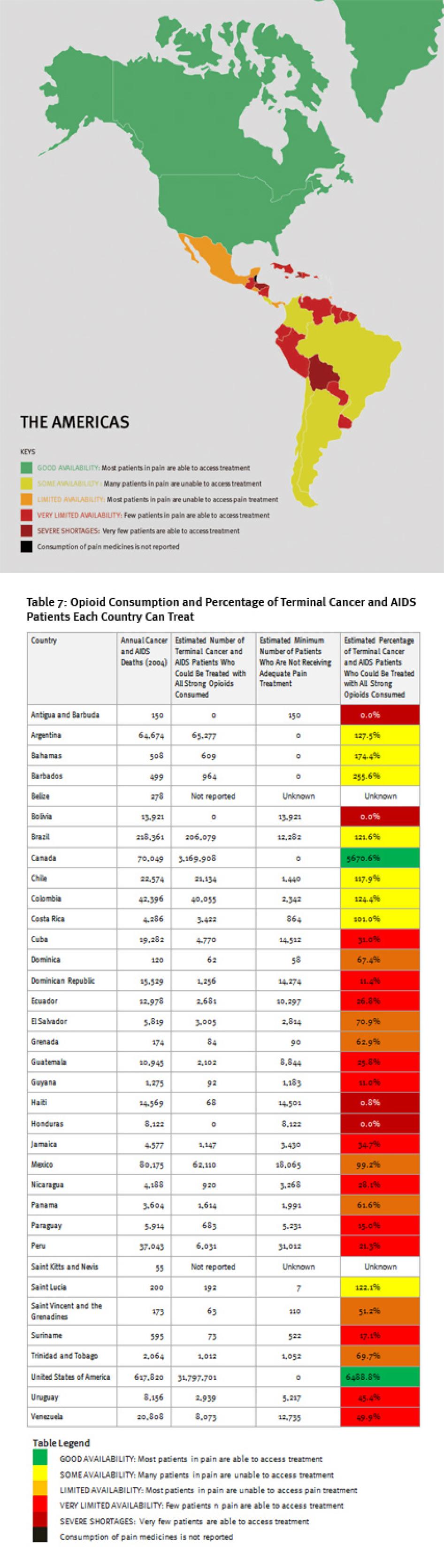
Map of The Americas
IV. The Americas
Regional Overview
"Cancer is killing us. Pain is killing me because for several days I have been unable to find injectable morphine in any place. Please, Mr. Secretary of Health, do not make us suffer any more."
– A classified ad placed in El País newspaper in Cali, Colombia, on September 12, 2008, by the mother of a woman with cervical cancer.
"[In the United States there is a] widely publicized chilling effect of physician prosecution on physicians concerned with legal scrutiny over prescribing opioids…regulators and law enforcement may do well to improve how they craft their public messages to physicians and how they handle routine investigations of medical practice.”
– Goldenbaum et al., "Physicians Charged with Opioid Analgesic-Prescribing Offenses," Pain Medicine, vol. 9, no. 6, 2008.
Consumption of opioid analgesics varies greatly in the Americas from some of the highest levels in the world in the United States and Canada to very low levels in Central America and the Caribbean. At least 100,000 terminal cancer and HIV/AIDS patients die without adequate pain treatment in the Americas each year, although the real number is probably much higher.
In Central America and the Caribbean, about half of the countries consume so few opioid medications that even if all were used exclusively to treat patients with terminal cancer and HIV for pain, less than a third of them could receive adequate treatment (Belize, El Salvador, Honduras, Nicaragua, Saint Kitts and Nevis, Trinidad and Tobago, Jamaica, Dominican Republic, and Haiti). Bolivia, Antigua and Barbuda, and Honduras reported no consumption of opioids for 2006 to 2008, and Haiti could treat pain in less than 1 percent of its terminal cancer and HIV/AIDS patients.
In South America, consumption levels are generally significantly higher than in Central America and the Caribbean countries, but still far lower than in North America or Western Europe. Several South American countries, such as Bolivia, Ecuador, Peru, and Suriname, significantly lag behind their neighbors. In these countries, even if all opioid medications were used exclusively to treat chronic pain, fewer than 40 percent of patients could be treated adequately.
Policy
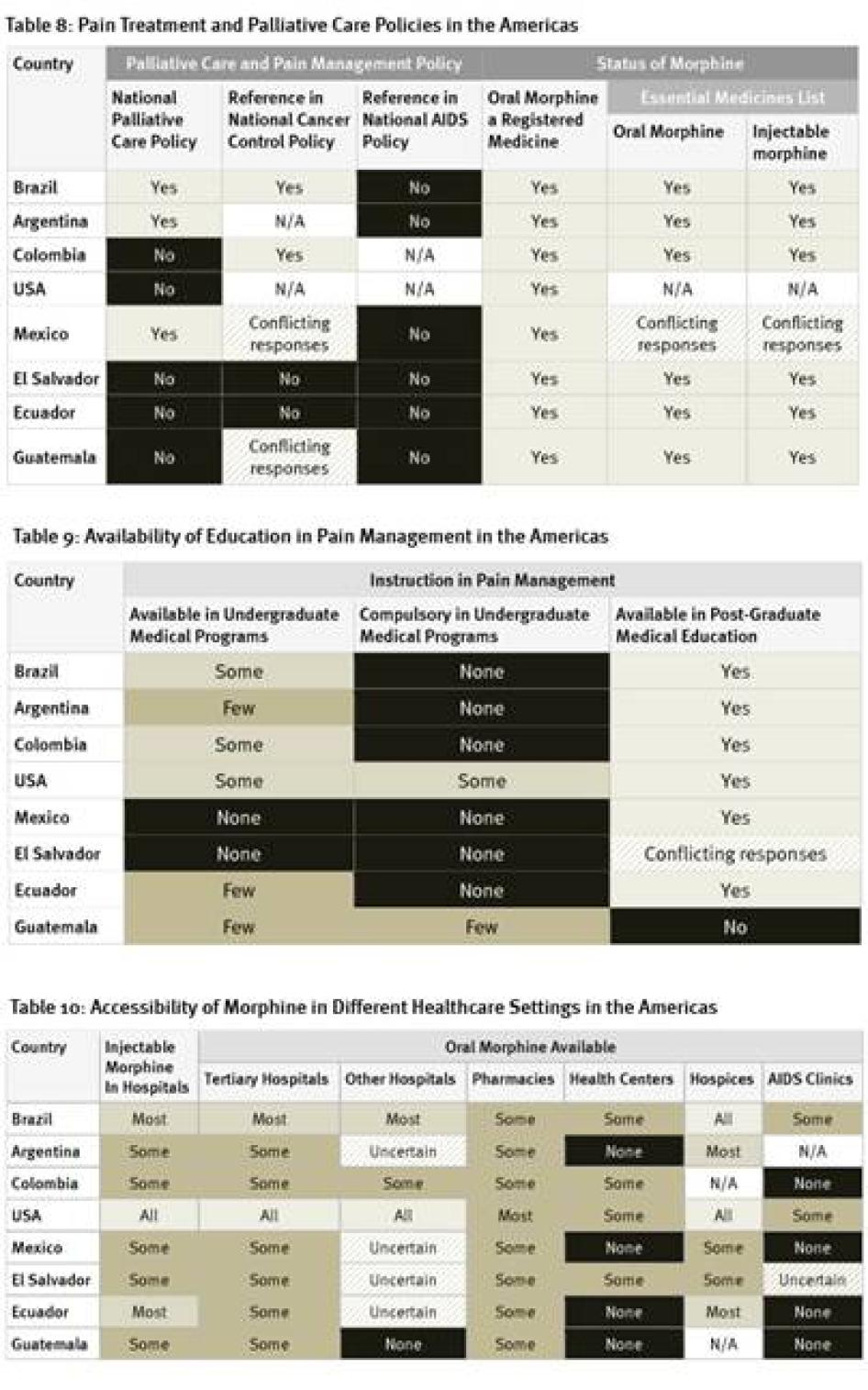
As Table 7 shows, policy support for palliative care is very limited in the countries surveyed in the Americas. Five of eight countries do not have national palliative care policies; survey participants in two countries that do have such policies, Argentina and Brazil, said that they are not implemented in practice.[92] A positive exception is Mexico, which recently adopted a policy on management of terminal patients. None of the countries surveyed have HIV policies that refer to palliative care and only two countries, Brazil and Colombia, address pain management in their national cancer control policies. More positively, oral morphine is a registered medicine in all countries surveyed, and most have it on their essential medicines lists.
Education
Availability of undergraduate education in pain management and palliative care is very scarce in the countries surveyed in the Americas. In two countries, Mexico and El Salvador, instruction on palliative care is altogether unavailable in undergraduate programs, while in most other countries it is available only in a few or some such programs. Instruction on palliative care is compulsory only in some undergraduate medical programs in the United States and in a few in Guatemala. All of the region’s larger countries have opportunities for post-graduate medical education in pain treatment or palliative care, but these are lacking in the less-populous countries, such as Guatemala and possibly El Salvador.
Drug Availability
Supply and Distribution
The United States, the country with by far the highest opioid consumption of countries surveyed, has the greatest availability of morphine across clinical settings, followed by Brazil. Guatemala had the poorest, with morphine available in only some pharmacies and tertiary hospitals. Throughout Latin America, only some pharmacies stock oral morphine. Its availability in health centers and HIV/AIDS clinics is even poorer. Survey respondents in all countries said that it is harder to access opioids outside major cities.
Drug Regulations
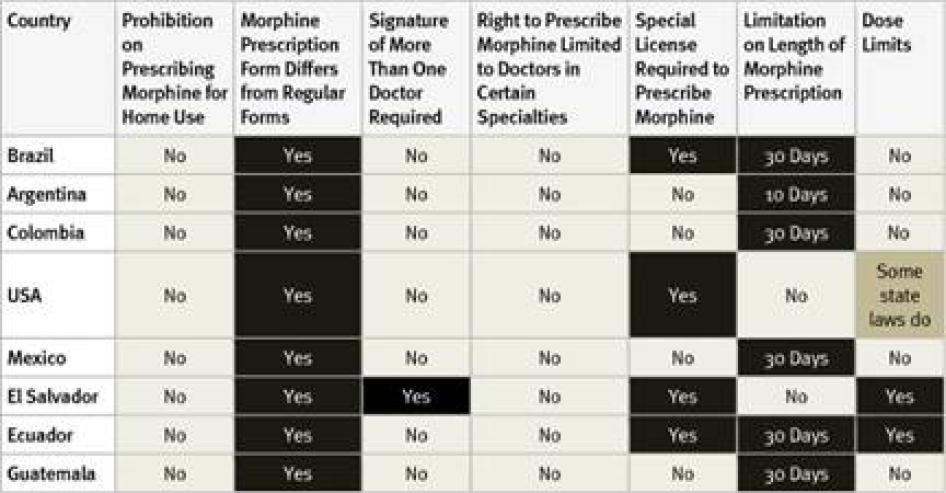
All countries surveyed in the Americas require special prescription forms and four require physicians to obtain a special license to be allowed to prescribe opioid medications. Guatemala, the country with the lowest opioid consumption of those surveyed, also imposed the most types of restrictive regulation, including dose limits. Most of the American countries surveyed, with the exception of the United States and El Salvador, also impose a limit on the number of days that a morphine prescription can cover. Five countries have a relatively generous 30-day limit. In Argentina, however, a prescription can cover just 10 days.
In El Salvador, all doctors can prescribe a limited, one-time dose of opioids to treat acute pain, but a different prescription form is needed to prescribe opioids for chronic pain, and those prescriptions must be authorized by the secretary of the health facility and the chief of the narcotics control agency. Survey respondents from all countries except Colombia said that healthcare workers fear legal sanction for mishandling opioids and that this was a deterrent to prescribing them. None of the Latin American countries surveyed allows nurse-prescribing. In most US states, some types of nurses can prescribe morphine. In a few states physician assistants or pharmacists can also prescribe but others impose dose limits.
Table 11: Restrictive Regulation of Morphine Prescribing in the Americas
Cost
Key informants in four countries—Argentina, Brazil, Colombia, and Mexico—said that the government subsidizes the cost of morphine in some circumstances. In Colombia, inexpensive oral liquid morphine is available, but in most countries surveyed in South America, most available morphine formulations are much more expensive, priced up to several dollars for a daily dose. In Ecuador, El Salvador, and Guatemala, the three countries with the lowest opioid consumption of those surveyed, inexpensive immediate release oral morphine is unavailable although costly sustained release tablets are, making the price of morphine unnecessarily high.
Best Practice and Reform Efforts: Colombia
In Colombia, intensive engagement between the government, NGOs, and academics has led to recent progress in improving access to palliative care and pain management services. In the last five years, the government has undertaken significant regulatory reforms to remove unnecessary barriers to accessing pain treatment and improve access to opioid medicines. In 2006 the government increased the maximum number of days allowed for the prescription of opioids from 10 to 30 days,[93] easing access for patients who need opioid therapy for extended periods of time. Revised regulation for regional drug procurement has also been put in place with the aim of improving opioid availability. The new regulation mandates all 32 Colombian states to have at least one place where opioids are guaranteed to be in stock at all times.[94] Morphine consumption has increased following these efforts to improve availability. Between 2006 and 2009, the government reported a 42 percent increase in units of morphine sold.[95]
Modest gains have also been made in the field of education. The country’s first mandatory course in palliative care for undergraduate medical students was implemented at the Universidad de la Sabana in Bogota and could serve as a model for other universities.[96] Continuing education for primary health workers in palliative care is also available to a limited extent.[97]
Columbia’s progress has resulted from several years of close engagement between the government and national and international NGOs and academic institutions. In 2006 members of the Universidad de la Sabana, the International Association for Hospice and Palliative Care, the Pain and Policy Study Group, and the University of Wisconsin developed an action plan for improving access to palliative care and pain management services in Colombia and later organized a workshop with members of the governments and the private health sector to identify barriers to accessing palliative care and solutions to these barriers.[98] These efforts have largely guided Columbia’s reform efforts.
Though Colombia still has far to go in guaranteeing access to pain treatment and palliative care for all who need it, greater progress may be on the horizon. The inclusion of three new opioid formulations in the country’s essential medicines list is being debated by the Regulatory Commission of Health.[99] In addition, a proposed law that would seek to improve access to controlled medicines, quality of palliative care services, and education for healthcare workers was drafted by two senators with input from several Colombian palliative care experts and organizations.[100] At time of writing, the senate had discussed the Bill but not yet voted on it.[101]
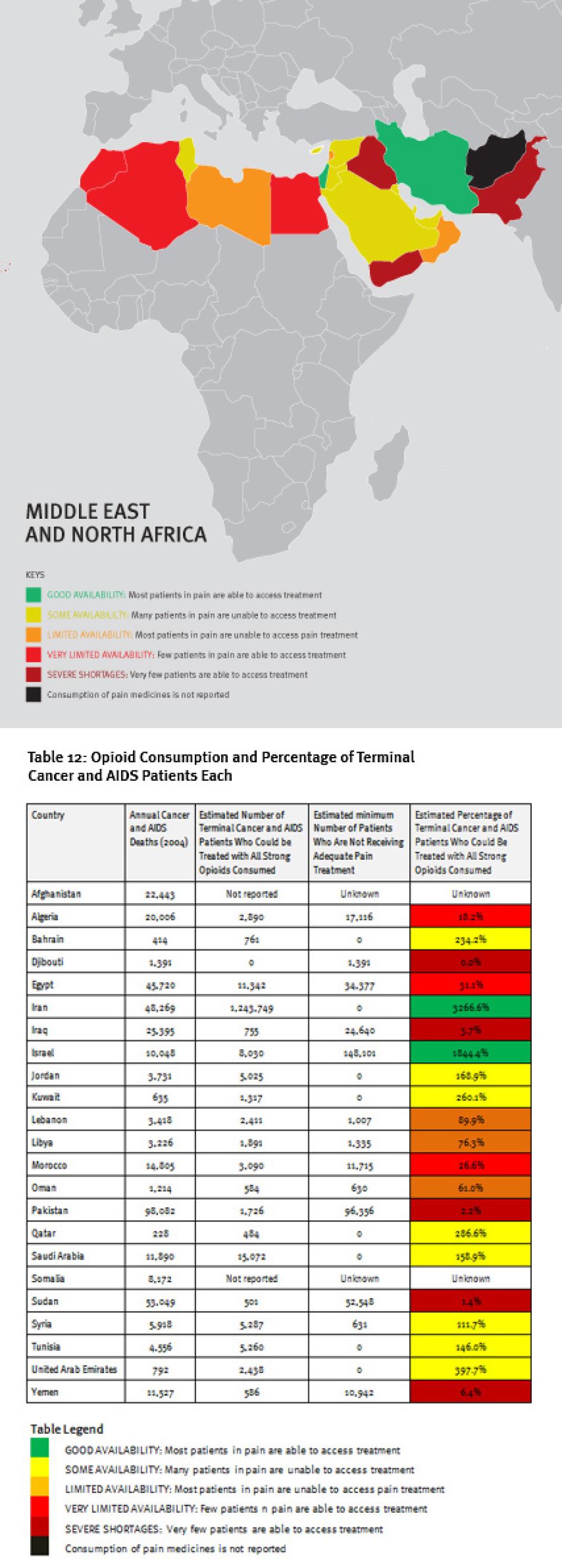
Map of the Middle East and North Africa
V. The Middle East and North Africa
Regional Overview
"Doctors are fearful of everything to do with opioids." – Oncologist, Jordan.
“[The prescription limitation of] seven days is not enough. It makes our work harder and forces patients to travel long distances to have access to morphine” – Professor of Oncology, Morocco.
The Middle East and North Africa region is characterized by vast differences in resources, containing some very poor and some very wealthy countries. These differences are clearly reflected in the availability of opioid analgesics. Four countries in the region—Iraq, Pakistan, Sudan, and Yemen—consume so few opioid medicines that even if all were used only to treat patients with terminal cancer and HIV/AIDS for pain, less than 10 percent of those patients could receive adequate pain treatment. Afghanistan and Somalia do not report opioid consumption to the INCB, and Djibouti reported no consumption for 2006 to 2008.
A number of oil-rich nations, such as Bahrain, Kuwait, Saudi Arabia, Qatar, and United Arab Emirates, also consume relatively few opioid medications. Iran stands out in the region for its high consumption of opioids, particularly methadone, but a significant proportion is used for treating drug dependence, not pain. In all, at least 180,000 patients in the region will die of cancer or HIV/AIDS each year without adequate pain treatment.
Policy
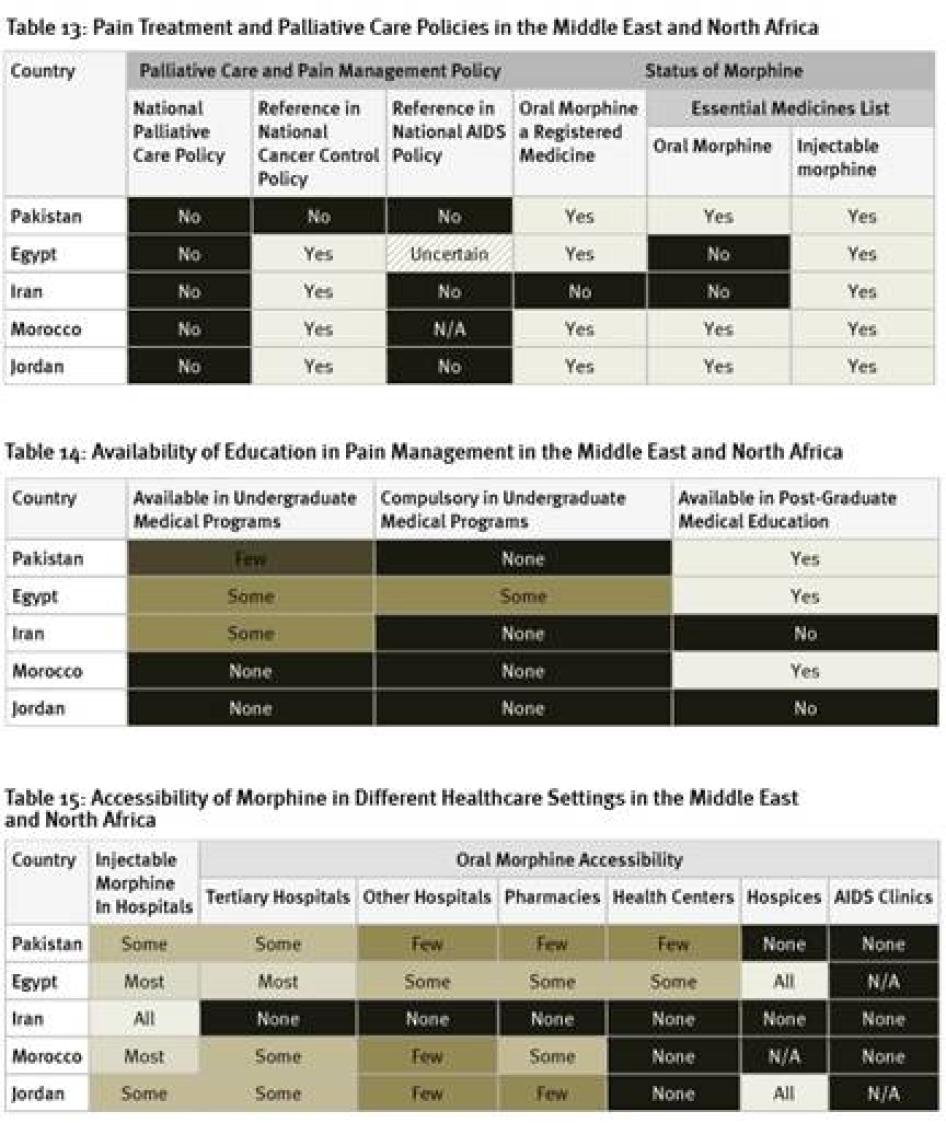
None of the countries surveyed from the region has a national palliative care policy, although survey respondents in Morocco expect one to be adopted soon. While the cancer control policies of four of the five countries surveyed include references to pain treatment or palliative care, HIV policies, where they exist, do not. Egypt and Iran are among just six of the forty countries surveyed that have not included oral morphine in their essential medicines list.[102] In fact, Iran is one of two of the forty countries surveyed where oral morphine is not a registered medicine and thus not available at all. Although an article in a peer-reviewed medical journal states that Iran’s cancer control policy covers palliative care, Iranian key informants we surveyed were unaware of this.[103]
Education
As shown in Table 14, Egypt is the only country surveyed in the region to have any compulsory instruction on palliative care as part of undergraduate medical programs. Morocco and Jordan do not have any instruction on palliative care available in such programs. In Iran and Jordan, no post-graduate instruction on palliative care exists.
Drug Availability
Supply and Distribution
While injectable morphine is available in most or all hospitals in Egypt, Iran, and Morocco, this is only the case in some hospitals in Jordan and Pakistan. The availability of oral morphine is particularly poor in the countries surveyed in the region. As mentioned above, in Iran it is altogether unavailable. In Pakistan, oral morphine is not available in any hospices and only in few pharmacies and health centers. In Jordan, while available in all hospices, no health centers have morphine and only few pharmacies. Of the countries surveyed, Egypt has the best availability of oral morphine, with the medication available in all hospices, most tertiary hospitals, and some pharmacies and health centers. Survey respondents from all countries surveyed said that it is harder to access morphine outside of major cities.
Drug Regulations
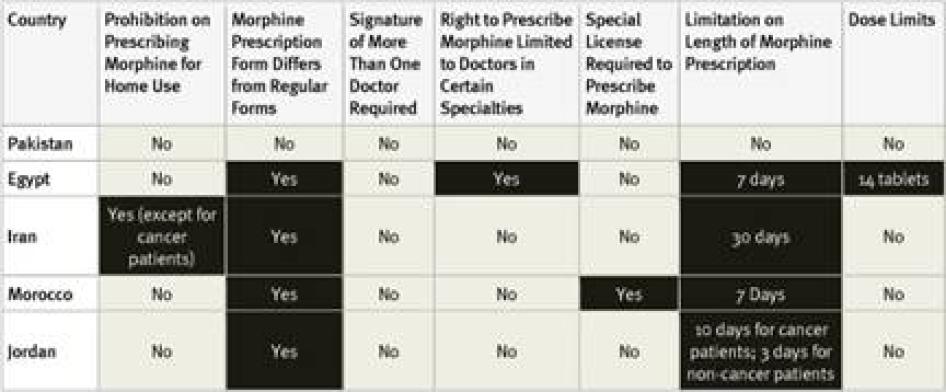
All countries surveyed in the region, except Pakistan, require special prescription forms for morphine. Survey respondents in Pakistan reported that some hospitals require the use of special prescription forms, even though they are not legally required. Four of the countries surveyed have limits on the length of time that a prescription can cover, again, all but Pakistan. In Iran, the limit is relatively generous at 30 days, but there are much shorter limits in Egypt (7 days), Jordan (10 days for cancer, 3 days for other patients), and Morocco (7 days).
Regulations also restrict who can prescribe morphine and to whom. In Iran, morphine can only be prescribed for home use for cancer patients. In Egypt, most doctors can only prescribe up to 14 morphine tablets. Only oncologists and pain specialists can prescribe more. In Morocco, general practitioners must obtain a license to prescribe morphine, while other doctors working in hospitals or larger clinics are covered by that facility’s license. In all of the counties surveyed, at least one respondent felt that fear of legal sanction was a deterrent to prescribing opioids. None of the countries surveyed allow nurse prescribing.
Table 16: Restrictive Regulation of Morphine Prescribing in the Middle East and North Africa
Cost
In the Middle East and North Africa, poor accessibility does not appear to be attributable to the cost of morphine. Respondents in all those countries surveyed reported that the morphine formulations available are generally inexpensive.
Developing Palliative Care: Jordan
Jordan is one country in this region that has made significant strides in the last decade in developing palliative care. Between 2001, when the reform efforts began, and 2008, the last year for which data is available, consumption of morphine increased 2.5 times. In 2004 the Jordanian government partially reformed drug regulations, removing a provision that limited morphine prescribing rights to oncologists and slightly increasing the number of days a prescription for cancer patients can cover from 3 to 10 days (although it remains 3 days for non-cancer patients). That same year, a local pharmaceutical company began producing low cost immediate release morphine tablets, leading to a significant decline in the costs of the medication and an increase in the number of formulations available.[104]
Although there is still no instruction on palliative care available in undergraduate or post-graduate medical programs in Jordan, some progress has been made in educating health professionals. Through international fellowships, a handful of physicians have received in-depth training. These physicians are now conducting palliative care training in other major oncology units in Amman and elsewhere.[105]
Jordan’s progress demonstrates how much is possible with government leadership and a coordinated effort by government agencies, healthcare workers, UN agencies, and civil society.
In 2001 the Jordanian government, WHO, healthcare workers, civil society, and the pharmaceutical industry came together and decided to establish the Jordan Palliative Care Initiative (JCPI), which was tasked with developing palliative care in the country and was designated a WHO Demonstration Project.[106] In November that year, the Jordanian Ministry of Health and WHO agreed to work together to better integrate palliative care into the Jordanian health system.[107] This lead to a joint workshop in 2003 to develop a national action plan and a National Palliative Care Committee.[108]
These achievements should embolden Jordan’s government to raise palliative care availability to the next level by developing and implementing a national palliative care policy, expanding availability of morphine to more health facilities, further reforming its drug regulations, and introducing palliative care instruction into undergraduate and postgraduate medical and nursing curricula.
Map of Europe
VI. Europe
Regional Overview
There is a clear pattern in opioid consumption in Europe: Western European countries all consume at least 10 times as many opioids as is necessary to treat their terminal cancer and HIV/AIDS patients; Eastern European countries consume less but generally several times more than is necessary to treat their terminal cancer and HIV/AIDS patients.
A handful of Eastern European and Central Asian countries—Armenia, Azerbaijan, Belarus, Kazakhstan, Russia, Ukraine, and Uzbekistan—consume only enough opioids to treat less than 30 percent of their terminal cancer and HIV/AIDS patients. Two central Asian countries, Tajikistan and Turkmenistan, can treat less than 10 percent of these patients. As a result, at least 480,000 terminal cancer and HIV/AIDS patients die in Europe each year without access to adequate pain treatment.
As mentioned, Western European countries all consume at least 10 times as many opioids as is necessary to treat their terminal cancer and HIV/AIDS patients, and some Western European countries—Austria, Denmark, Germany, and Switzerland—consume more than thirty times more. These medicines are necessary to treat the many other patients that suffer pain, and this tdemonstrates that comparing actual opioid consumption to that which is necessary to treat terminal cancer and HIV/AIDS patients only gives an indicator of a country’s relative ability to meet its patients’ needs for pain relief.
In countries where most people have access to the medicines they need most of the time, consumption is much higher than that which is necessary to treat terminal cancer and HIV/AIDS patients. Countries who can treat only those patients (i.e. those that score around 100 percent in these tables, such as Chile, Costa Rica, Mexico, Syria, Lebanon, and Uzbekistan) still have a very long way to go to ensure all patients in need can access essential pain medicines.
Our survey results indicate that the marked contrast between opioid consumption in Eastern and Western Europe is not solely attributable to differences in economic development or medical infrastructure. The Eastern European countries surveyed have fewer policies to support pain treatment and palliative care, fewer opportunities for education in pain management and palliative care, more restrictive regulation on prescribing, and poorer accessibility of morphine across a range of healthcare settings.
Policy
Governments in four of the nine countries surveyed in Europe provide strong policy support for palliative care: France, Poland, Turkey, and the United Kingdom all have national palliative care policies and cancer control policies that include palliative care. The first three also have national HIV policies that reference palliative care. There has been recent progress in Georgia, where a national palliative care policy and a cancer policy that includes a palliative care development plan have been submitted to parliament for adoption, but the HIV policy makes no reference to palliative care. Other countries surveyed provide less policy support. Germany does not have a national palliative care policy nor a national cancer or HIV control policy or an essential medicines list. Romania, Russia, and Ukraine do not have national palliative care policies but do provide for palliative care in national cancer control plans.
Ukraine stands out as the only country surveyed in Europe where oral morphine is not a registered medicine. Ukraine and Georgia are the only countries surveyed that have essential medicines lists but have not included oral morphine.
Table 18: Pain Treatment and Palliative Care Policies in Europe
Education
The European countries surveyed have the most extensive availability of training in pain management of any region. In France, Poland, and the United Kingdom, training in palliative care is compulsory for all undergraduate medical students. In 2009 Germany introduced legislation that will make training in palliative care compulsory for all undergraduate medical students by 2014. In all other countries surveyed, with the exception of Russia, palliative care instruction was available in at least some undergraduate medical programs. In Russia such instruction is available only in a few such programs. Survey respondents from all countries said that training in palliative care is available in post-graduate medical education.
Table 19: Availability of Education in Pain Management in Europe
Drug Availability
Supply and Distribution
Our survey findings show a large gap between availability of morphine in Western and Eastern Europe. Availability was best in France, Germany, and the United Kingdom, with oral and injectable morphine available in most or all hospitals and pharmacies. In Poland and Turkey, the medications are available in most or all hospitals but only in some pharmacies. Survey respondents in Romania and Russia said injectable morphine was available in most hospitals but oral morphine only in some hospitals and pharmacies. Georgia and Ukraine reported the most problematic situations, with oral morphine altogether unavailable in Ukraine and only available in some tertiary hospitals, pharmacies, and hospices in Georgia. In most countries surveyed, except Germany, Poland, and France, survey respondents reported that it is harder to access morphine outside of major cities.
Table 20: Accessibility of Morphine in Different Healthcare Settings in Europe
Drug Regulations
Significant differences between regulations in Western and Eastern Europe are apparent from the survey, with Georgia, Russia, and Ukraine imposing both greater numbers of and more severe restrictive prescription requirements than other countries surveyed. Four of five European Union countries require special prescription forms but do not impose other problematic regulatory restrictions.
Turkey requires special prescription forms, as well as signatures from multiple doctors for long-term opioid prescriptions, and imposes a 200 mg daily dose limit for morphine, which is likely to be insufficient for significant numbers of patients. Georgia, Russia, and Ukraine require special prescription forms but doctors must also get special licenses to be allowed to prescribe morphine, and these counties impose low limits on the number of days a prescription can cover. Russia and Ukraine also require that multiple doctors sign prescriptions and impose limits on daily doses, with Ukraine’s limits particularly low, lower than the average daily dose for a patient with terminal cancer of HIV/AIDS.[109] Georgia and Ukraine also limit the right of prescription of opioids to doctors in certain specialties, such as oncology. In all surveyed countries except Romania and France, survey respondents reported that fear of legal sanction deters prescribing of opioids.
Table 21: Restrictive Regulation of Morphine Prescribing in Europe
Cost
In most of the European countries surveyed, healthcare workers mentioned that the cost of morphine is fully or partially-subsidized by the government, but in several countries only cancer patients qualify for subsidies or subsidies are more generous for cancer patients.
Best Practice and Reform Efforts: Romania
Romania has taken significant steps in the last few years to revise laws and regulations in order to improve access and availability of pain treatment and palliative care in the country. New legislation from November 2006 and new regulations from May 2006 corrected imbalanced laws and regulations that had severely limited doctors’ authority to prescribe opioids in Romania. The new laws and regulations establish that it is the sole responsibility of the doctor to determine the appropriate opioid dose and allow doctors to prescribe opioids to patients in severe pain regardless of the underlying disease. Prior to the reforms, doctors could only prescribe opioids to patients suffering from a limited class of diseases, such as cancer in its advanced stages. Progress has also been made in ensuring the availability of a variety of opioid formulations in Romania, many of which are produced domestically.
The new regulations also provide for the improvement of Romanian health workers’ education in palliative care.[110] The new regulations led Casa Sperantei, the first independent hospice in Romania, in Brasov, to offer courses in palliative care to health professionals, with the assistance of a grant from the Open Society Institute (OSI). Over 4,000 doctors have since completed this Ministry of Health-approved course. Although the Romanian Ministry of Health had identified palliative care as a “medical sub-specialty” in 2000, the medical profession had not received adequate training, and fear of prescribing opioids on the part of health professionals remains widespread. Increased and improved education in palliative care is a step towards addressing these barriers.
Romania’s progress demonstrates what can be achieved through the combined efforts of local professionals, international experts, and national authorities.[111] Reform efforts in Romania began with Romania’s participation in a 2002 workshop convened by OSI and WHO. Following an initial assessment and action plan for improving palliative care in Romania, Romania was selected as a pilot country by OSI and the Pain and Policy Studies Group (PPSG). In 2002, the Romanian Ministry of Health appointed a commission of specialists to provide reform recommendations. Working in collaboration with PPSG, the palliative care commission used WHO guidelines to present the Ministry of Health with a number of recommendations for the reform, many of which were incorporated into the country’s revised laws and regulations.
Despite the impressive progress that has been made, substantial challenges remain in providing palliative care in Romania. Many hospitals continue not to stock morphine because it “is not included in the drug list for emergencies in acute hospitals.” This leads to reliance on pethidine, a weaker opioid that is not appropriate for treatment of chronic pain. Accessibility issues persist as the number of pharmacies stocking morphine, particularly in rural areas, remains inadequate. Many doctors in Romania are still hesitant to prescribe opioids, indicating that further education efforts are needed.[112]
Map of Asia
VII. Asia
Regional Overview
"My leg would burn like a chili on your tongue. The pain was so severe I felt like dying. I was very scared. I felt that it would be better to die than to have to bear this pain. [I thought], just remove the leg, then it will be alright. Just get rid of the leg so I'll be free of pain."
- Dilawar Joshi, a Nepali man with a bone tumor, India
"I would sleep maybe an hour and a half per night. I could take any number of sleeping pills [without effect]. With morphine, I can relax. This place [the palliative care unit] is heaven-sent..."
- Shruti Sharma, Hyderabad, a breast cancer patient, India
Consumption of opioids varies dramatically throughout Asia. Australia and New Zealand consume more than 20 times more opioids than are needed to treat their terminal cancer and HIV/AIDS patients. By contrast, Bhutan, North Korea, India, Indonesia, the Maldives, Mongolia, Nepal, and Sri Lanka can all treat less than 20 percent of their terminal cancer and HIV/AIDS patients, and Bangladesh, Burma, Cambodia, Laos, and Vietnam less than 10 percent. The Solomon Islands reported no consumption of opioids at all between 2006 and 2008.
The world’s two most populous countries—India and China—can treat just 12 and 53 percent of their terminal cancer and HIV/AIDS patients respectively. Thus although Asia has better treatment coverage than sub-Saharan Africa, it also has the largest number of patients suffering without treatment of any region, at least 1.7 million terminal cancer and HIV/AIDS patients.
Policy
While nine of eleven countries surveyed reference palliative care in their national cancer control policies (Cambodia and Nepal are the exceptions) only four of eleven—Indonesia, Philippines, South Korea, and Vietnam—have national palliative care policies. Only three countries— Cambodia, Nepal, and Vietnam—provide for palliative care in national HIV policies. Oral morphine is a registered medicine in all the countries surveyed, and both oral and injectable formulations are on the essential medicines lists of all countries except South Korea. Vietnam stands out as the country that provides the best policy support for palliative care, with a national palliative care policy and palliative care provisions in its national cancer and HIV plans.
Table 23: Pain Treatment and Palliative Care Policies in Asia
Education
Availability of instruction on palliative care in undergraduate medical programs is poor in most countries in the region surveyed, with Bangladesh and Nepal, which both offer compulsory instruction on palliative care in most undergraduate medical programs, performing best. Palliative care instruction is available only in few undergraduate programs in India, Indonesia, and South Korea. In Cambodia, Philippines, South Korea, and Vietnam, no compulsory instruction in palliative care is available in any undergraduate medical programs. Key informants in most countries said that instruction on palliative care was available in post-graduate medical education, but in China such instruction is not available at all.
Table 24: Availability of Education in Pain Management in Asia
Drug Availability
Key informants in Cambodia, China, Japan, and South Korea reported the widest availability of morphine, with the medication available in most or all hospitals. However, in Cambodia and China, oral morphine is not available at all in pharmacies and in few or no health centers, creating significant obstacles to its accessibility in rural areas. Poorest overall availability was reported in Bangladesh, where oral morphine is not at all available in hospitals and health centers and only in some pharmacies and few hospices in India, Nepal, Philippines, and Vietnam. In each of these countries oral and injectable morphine is available only in some hospitals, health centers, and pharmacies, although reported availability in hospices is slightly better. Doctors in all the countries surveyed in Asia reported that morphine is harder to access outside major cities.
Table 25. Accessibility of Morphine in Different Healthcare Settings in Asia
Medicines Availability: Restrictive Regulation
Of the 11 Asian countries surveyed, Cambodia has by far the most restrictive regulations. It prohibits prescribing of morphine for home use, requires doctors to get a special license to be allowed to prescribe morphine, requires multiple doctors to sign off on morphine prescriptions, and imposes a 7-day limit on the number of days a prescription can cover.
Seven of the ten other Asian countries surveyed require a special prescription form for morphine—India, Indonesia, and Nepal are the exceptions—and three others, China, Japan, and Philippines, require doctors to obtain a special license before they can write such prescriptions. Regulations in four of the other ten countries imposed limitations on the number of days a prescription can cover. In Japan, Philippines, and Vietnam, the limit is a relatively generous 30 days. In China, it depends on the formulation of morphine: 15 days for sustained release, 7 days for immediate release, and 3 days for injectable morphine. Survey respondents in South Korea, where regulations do not impose such limit, said that many hospitals enforce their own limits, which are often as short as one week or even one day. In all but two of the countries surveyed in the region—Japan and Thailand—doctors reported that fear of legal sanction deters opioid prescribing.
None of the countries surveyed in Asia allow nurse-prescribing, although Vietnam allows assistant doctors to prescribe morphine in remote areas (see below).
Table 26. Restrictive Regulation of Morphine Prescribing in Asia
Cost
The cost of morphine varies markedly throughout the region. The price can be very inexpensive in countries that have domestic production of morphine, including India and Vietnam, and other countries, including Japan and China, subsidize its cost. In some countries, including Nepal, there is an official government price for morphine, but shortages mean the price on the black market is sometimes much higher.
Developing Palliative Care: Vietnam
Between 2005, when palliative care reforms began, and 2008, Vietnam saw an over 800 percent increase in morphine equivalent consumption, from 0.3 mg per person to 2.5 mg per person.[113] Vietnam has focused its reform efforts on removing unnecessary barriers to prescribing opioids and educating healthcare personnel in palliative care. In 2008 the country eased a number of key regulatory barriers to opioid prescription: the maximum daily opioid dose was abolished, prescriptions can now be issued for 30 days rather than 7, and district hospitals and commune health posts are now authorized to prescribe and dispense morphine.[114] Assistant doctors in “mountainous, remote, island, disadvantage areas and places where a doctor is not available” are also now able to obtain a license to prescribe morphine.[115]
New education programs and opportunities have also been developed. In 2008 the Ministry of Health piloted a certification program in palliative care and held a two-day workshop on new palliative care guidelines and regulations for more than 1,000 health care managers, pharmacists, and physicians from around the country.[116] As of 2010, 400 Vietnamese doctors have completed a one-week curriculum in palliative care, developed with assistance from the Harvard Medical School Center for Palliative Care.[117] Two Vietnamese medical colleges now offer instruction on palliative care to undergraduate medical and nursing students, and a National Curriculum in Palliative Care was expected to be published in 2010.[118]
This progress started with the creation of a working group on palliative care, which consisted of Ministry of Health officials, cancer and infectious disease physicians, and experts from NGOs supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR). The working group decided to conduct a rapid situation analysis to assess the availability of and the need for palliative care in Vietnam.[119] Based on the rapid situation analysis’s findings, the working group recommended that national palliative care guidelines and a balanced national opioid control policy be developed, training for healthcare workers be expanded, and that availability and quality of palliative care services be improved at all levels. In September 2006, the Ministry of Health issued detailed Guidelines on Palliative Care for Cancer and AIDS Patients, which provided guidance to practitioners on palliative care and pain management, and in February 2008, it issued new guidelines on opioid prescription, which eased regulatory barriers as described above.
Despite this progress, numerous challenges remain in delivering palliative care in Vietnam. Attitudes toward, and an understanding of, palliative care among health care professionals continue to be limited and lag behind regulatory changes. Although morphine can be prescribed for 30 days, a prescription can only be filled for 10 days a time, after which point it must be confirmed that the patient is alive and using the medication appropriately.[120] The availability of opioids continues to be limited, especially in rural areas, as few pharmacies and hospitals stock oral morphine.
VIII. International Human Rights Obligations and Pain Treatment
Health as a Human Right
The right to the highest attainable standard of health is a fundamental human right enshrined in numerous international instruments.[121] The International Covenant on Economic, Social and Cultural Rights (ICESCR) specifies that everyone has a right “to the enjoyment of the highest attainable standard of physical and mental health.”[122] The Committee on Economic, Social and Cultural Rights (CESCR), the body charged with monitoring compliance with the ICESCR, has held that states must make available in sufficient quantity “functioning public health and health-care facilities, goods and services, as well as programmes” and that these services must be accessible.[123]
Because states have different levels of resources, international law does not mandate the standard of health care to be provided. Rather, the right to health is considered a right of “progressive realization.” By becoming party to the international agreements, a state agrees “to take steps … to the maximum of its available resources” to achieve the full realization of the right to health. In other words, high income countries will generally have to provide healthcare services at a higher level than those with limited resources. But all countries will be expected to take concrete steps towards increased services, and regression in the provision of health services will, in most cases, constitute a violation of the right to health. The CESCR has held that states have a “specific and continuing obligation to move as expeditiously and effectively as possible towards the full realization” of the right to health and must “refrain from interfering directly or indirectly with [its] enjoyment.”[124]
The CESCR has called for an integrated approach to the provision of “preventive, curative and rehabilitative health treatment,”[125] which “should not disproportionately favour expensive curative health services which are often accessible only to a small, privileged fraction of the population.”[126] The committee has specifically called for “attention and care for chronically and terminally ill persons, sparing them avoidable pain and enabling them to die with dignity.”[127] States must refrain from actions that interfere with access to palliative care and take reasonable steps to facilitate its development and its integration into the health care system as a whole.
But the CESCR has also held that there are certain core obligations that are so fundamental that states must fulfill them. While resource constraints may justify only partial fulfillment of some aspects of the right to health, the committee has observed vis-à-vis the core obligations that “a State party cannot, under any circumstances whatsoever, justify its non-compliance with the core obligations… which are non-derogable.”[128] The committee has identified, among others, the following core obligations:
- To ensure the right of access to health facilities, goods and services on a non-discriminatory basis, especially for vulnerable or marginalized groups;
- To provide essential drugs, as from time to time defined under the WHO Action Programme on Essential Drugs;
- To ensure equitable distribution of all health facilities, goods and services;
- To adopt and implement a national public health strategy and plan of action, on the basis of epidemiological evidence, addressing the health concerns of the whole population.[129]
Relevant obligations of “comparable priority” include: ensuring child health care; taking measures to treat and control epidemic and endemic diseases; providing education and access to information for important health problems; and providing appropriate training for health personnel.[130] The CESCR has also stressed the “obligation of all States parties to take steps, individually and through international assistance and cooperation … towards the full realization of the rights recognized in the Covenant, such as the right to health.”[131]
Pain Treatment and the Right to the Highest Attainable Standard of Health
As morphine and codeine are on the WHO Model List of Essential Medicines, countries must provide these medications as part of their core obligations under the right to health, regardless of whether they have been included on their domestic essential medicines lists.[132] They must make sure that they are both available in adequate quantities and physically and financially accessible for those who need them.
Because the manufacturing and distribution of controlled medicines including morphine and codeine are entirely within government control, states need to put in place an effective procurement and distribution system and create a legal and regulatory framework that enables health care providers in both the public and private sector to obtain, prescribe and dispense these medications. Any regulations that arbitrarily impede the procurement and dispensing of these medications will violate the right to health.
States need to adopt and implement a strategy and plan of action for the roll-out of pain treatment and palliative care services. Such strategy and plan of action should identify obstacles to improved services as well as steps to eliminate them. States should regularly measure progress made in ensuring availability and accessibility of pain relief medications.
The requirement of physical accessibility means that pain medications must be “within safe physical reach for all sections of the population, especially vulnerable or marginalized groups, such as … persons with HIV/AIDS.”[133] This means that states should ensure that a sufficient number of health care providers or pharmacies stock and dispense morphine and codeine and that an adequate number of healthcare workers are trained and authorized to prescribe these medications.
Financial accessibility means that, while the right to health does not require states to offer medications free of charge, they must be “affordable for all.”[134] In the words of the CESCR:
Payment for health-care services…has to be based on the principle of equity, ensuing that these services, whether privately or publicly provided, are affordable to all, including socially disadvantaged groups. Equity demands that poorer households should not be disproportionately burdened with health expenses as compared to richer households.[135]
Countries also have an obligation to progressively implement palliative care services, which, according to WHO, must have “priority status within public health and disease control programmes.”[136] Countries need to ensure an adequate policy and regulatory framework, develop a plan for implementation of these services, and take all steps that are reasonable within available resources to execute the plan. Failure to attach adequate priority to developing palliative care services within health care services will likely violate the right to health.
Pain Treatment and the Right to Be Free from Cruel, Inhuman, and Degrading Treatment
The right to be free from torture, cruel, inhuman, and degrading treatment or punishment is a fundamental human right that is recognized in numerous international human rights instruments.[137] Apart from prohibiting the use of torture, cruel, inhuman, and degrading treatment or punishment, the right also creates a positive obligation for states to protect persons in their jurisdiction from such treatment.[138]
As part of this positive obligation, states have to take steps to protect people from unnecessary pain related to a health condition. As the UN Special Rapporteur on torture, cruel, inhuman and degrading treatment and punishment wrote in a joint letter with the UN Special Rapporteur on the right to health to the commission on narcotic drugs in December 2008:
Governments also have an obligation to take measures to protect people under their jurisdiction from inhuman and degrading treatment. Failure of governments to take reasonable measures to ensure accessibility of pain treatment, which leaves millions of people to suffer needlessly from severe and often prolonged pain, raises questions whether they have adequately discharged this obligation.[139]
In a report to the Human Rights Council, Manfred Nowak, then-special rapporteur on torture, cruel, inhuman and degrading treatment and punishment, specified that “the de facto denial of access to pain relief, if it causes severe pain and suffering, constitutes cruel, inhuman or degrading treatment or punishment” and that “all measures should be taken to … overcome current regulatory, educational and attitudinal obstacles to ensure full access to palliative care.”[140]
Not every case where a person suffers from severe pain but has no access to appropriate treatment will constitute cruel, inhuman, or degrading treatment or punishment. Human Rights Watch believes that this will only be the case when the following conditions are met:
- The suffering is severe and meets the minimum threshold required under the prohibition of torture and cruel, inhuman, or degrading treatment;
- The state is, or should be, aware of the level and extent of the suffering;
- Treatment is available to remove or lessen the suffering but no appropriate treatment is offered; and
- The state has no reasonable justification for the lack of availability and accessibility of pain treatment.
In such cases, states will be liable for failing to protect a person from cruel, inhuman, or degrading treatment.
IX. Recommendations
The palliative care and pain treatment gap is an international human rights crisis that needs to be addressed urgently both at the international and national level. Therefore, Human Rights Watch makes the following recommendations:
To Governments around the World
General
- Establish, where this has not yet been done, a working group on palliative care and pain management. This working group should include all relevant actors, including health officials, drug regulators, health care providers, nongovernmental palliative care providers, and academics, and develop a concrete plan of action for the progressive implementation of pain treatment and palliative care services.
- Assess both the availability of and the need for pain management and palliative care services.
- Develop a comprehensive plan of action that addresses the various barriers that impede availability of pain management and palliative care, including government policy, education, and availability of medications.
- Invite the WHO Access to Controlled Medications Programme to assist in implementing the above recommendations.
- National human rights commissions or ombudsman offices should, where possible, investigate obstacles to availability of pain management and palliative care services, and request that their governments take urgent measures to address them.
Ensuring an Effective Supply System
- Submit, in a timely fashion, realistic estimates for the need of controlled medications to the INCB.
- Ensure an effective distribution system for controlled medications. While procurement, transportation, and stocking regulations should be able to prevent potential abuse, they should not arbitrarily complicate these processes.
- Countries must ensure that in each region at least a minimum number of pharmacies and hospitals stock morphine.
Developing and Enacting Pain Management and Palliative Care Policies
- Recognize a human rights obligation to provide effective/adequate palliative care programs.
- Develop official policies on pain management and palliative care, including as part of cancer and HIV/AIDS control programs.
- Develop practical guidelines on pain management and palliative care for healthcare workers.
- Include oral morphine and other essential pain treatment medications in national lists of essential medicines.
- Ensure that drug control laws and regulations recognize the indispensible nature of opioid and other controlled medicines for the relief of pain and suffering, as well as the obligation to ensure their adequate availability.
Ensuring Instruction for Healthcare Workers
- Ensure adequate instruction for healthcare workers, including doctors, nurses, and pharmacists, at both undergraduate and postgraduate level.
- Instruction should also be offered to those already practicing as part of continuing medical education.
Reforming Drug Regulations
- Review drug control regulations to assess whether they unnecessarily impede accessibility of pain medications. Health care providers should participate in conducting this review.
- If regulations are found to impede access, they should be amended.
- Recommendations of WHO and health care providers should be a starting point of revised drug control regulations.
- Requiring special licenses for health care institutions or providers to handle morphine should be avoided as much as possible. In other cases, transparent and simple procedures should be established for obtaining such special licenses.
- Special prescription procedures for controlled medications should be avoided as much as possible. Where they are nonetheless in place, they should be minimally burdensome.
- Limitations on the amount of morphine that can be prescribed per day should be abolished.
- Unnecessary limitations on the amount of morphine that can be prescribed or dispensed at once should be abolished.
Ensuring Affordability of Medications
- Countries should seek to ensure the affordability of morphine and other opioid analgesics.
To Global Drug Policy Makers
- Restore the balance between ensuring availability of controlled medicines and preventing abuse, as provided for by the UN drug control conventions, in global drug policy debates. Access to controlled medicines should be a central and recurring agenda item at the Commission on Narcotic Drugs and other meetings on global drug policy.
- The Commission on Narcotic Drugs and the INCB should regularly review progress made by countries toward adequate availability of pain treatment medicines, carefully analyzing steps taken to advance this important issue.
- INCB should significantly increase its efforts to encourage and assist states in improving availability of opioid analgesics.
- UNODC should amend the model laws and regulations it has developed to include recognition of the indispensible nature of narcotic drugs and psychotropic substances for medical and scientific purposes and the obligation for states to ensure their availability.
- UNODC and the INCB should develop processes to ensure that human rights, including the right to the highest attainable standard of health, are systematically considered in their work.
To the WHA, WHO, UNAIDS, and the Donor Community
- The World Health Assembly (WHA) should adopt a resolution calling upon its member states to promote universal access to pain management and palliative care, by taking steps to ensure that their health policies and services address the needs of all patients with life-limiting illness, including by ensuring the availability of pain medicines and training in pain treatment and palliative care for healthcare workers.
- WHO should continue to treat access to controlled medicines with urgency through its Access to Controlled Medications Programme.
- Donor countries and agencies, including the Global Fund to fight AIDS, Malaria, and Tuberculosis and the U.S. President’s Emergency Plan for AIDS Relief, should actively encourage countries to undertake comprehensive steps to improve access to pain relief medications and support those that do, including through support for the WHO Access to Controlled Medications Programme.
- UNAIDS should work with governments to identify and remove obstacles to availability and accessibility of pain management and palliative care services.
To the Global Human Rights Community
- UN and regional human rights bodies should routinely remind countries of their obligation under human rights law to ensure adequate availability of pain medicines.
- UN human rights procedures that monitor the right to the highest attainable standard of health and the prohibition on cruel, inhuman and degrading treatment should regularly consider the availability of pain medicines and governments’ efforts to make them available.
- Human rights groups should include access to pain treatment and palliative care into their work, including by submitting shadow reports to UN treaty bodies, providing information to the UN Special Rapporteurs on the highest attainable level of health and on torture, cruel, inhuman and degrading treatment and punishment to the Human Rights Council.
X. Methodology
This report presents information on barriers to accessing pain treatment in 40 countries.
Countries were selected in two steps to ensure a broad and diverse sample. First, the five most populous countries in each of the sixWHO regions were chosen. Second, specific countries were selected where additional diversity of experience or in-depth understanding of experience was desired.
In Europe, 10 countries were selected in order to include a range of countries from both Western Europe and formerly Communist countries of Eastern and Central Europe. Countries in different regions (including Cameroon, Uganda, El Salvador, Ecuador, Guatemala, Jordan, Georgia, and Cambodia) where Human Rights Watch had on-going work were also included.
From this overall list of countries, four countries were excluded because collecting information was impractical or constituted an unacceptable level of risk for the healthcare workers (the Democratic Republic of Congo, Uzbekistan, Burma, and Sudan). In their place, the next-most-populous country in the relevant WHO region was chosen. Among selected countries, one (Italy), was excluded after repeated efforts to contact appropriate survey respondents yielded no responses.
The primary means of collecting this data was surveying healthcare workers by telephone interview. The survey questions (see Annex 2) ask about common barriers to access to pain treatment identified in Human Rights Watch’s March 2009 report: “Please, do not make us suffer any more…”: Access to Pain Treatment as a Human Right. The telephone interviews took place between July 2009 and October 2010.
The healthcare workers interviewed for this research were identified through professional associations and nongovernmental organizations that work on access to pain treatment and palliative care. Two survey respondents were interviewed in each country except Guatemala, China, and Cambodia, where there were three survey respondents. Most survey respondents (77 of 82) were medical doctors, many of whom also held academic appointments. Three survey respondents were nurses, one was the head of a national hospice and palliative care association with experience as a hospice administrator, and one was a technical advisor for a nongovernmental organization working to integrate palliative care into the country’s health system. Many of the healthcare workers were palliative care specialists; others were specialists in pain management, anesthesiology, or oncology.
Healthcare workers were initially contacted by email, with a description of the project and the survey questions. Healthcare workers who agreed to participate were then interviewed by telephone to collect their survey responses. At the preference of the respondent, interviews were conducted in English, Spanish, French, Russian, or Mandarin. In addition, internet research was used to gather secondary materials relevant to the survey questions, such as national palliative care policies, cancer and HIV/AIDS control policies, essential medicines lists, and drug control laws and regulations.
The healthcare workers’ survey responses and the results of the secondary research were compared and the healthcare workers were then contacted by email seeking clarification of discrepancies between the survey answers they each provided or between their answers and any relevant documents collected. Survey respondents from 35 countries responded with clarifying information.
Once clarifying information was received, letters presenting the survey results were sent to the Ministry of Health and the Competent National Authority—the body responsible for implementing the 1961 Single Convention on Narcotic Drugs—in each country. The letters explained the research and invited clarification of the initial research findings or additional relevant information. The letters were sent by post and, where possible, fax or email. Replies were received from Poland, Jordan, Georgia, Uganda, and El Salvador.
The initial survey results were also published on a password protected website. Through the email newsletters of the Worldwide Palliative Care Alliance and the International Association for Hospice and Palliative Care, members of these organizations were invited to comment on the initial findings with clarifications or additional relevant information.
The maps and tables of opioid consumption in this report were prepared using publically available data to compare the availability of medicines to treat moderate to severe pain in countries around the globe.[141] Data on each country’s consumption of the principal medicines used to treat moderate to severe pain is published each year by the International Narcotics Control Board.[142] Using expert estimates of the prevalence and severity of pain in terminal cancer and HIV/AIDS patients,[143] and WHO data on cancer and HIV/AIDS mortality,[144] a calculation of each country’s ability to provide pain treatment for its terminal cancer and HIV/ AIDS patients was made, as an indicator of the availability of treatment for all patients with moderate to severe pain in the country. A table of relevant data and calculations can be found in appendix 3.
Survey responses are presented in five regional chapters: Africa, Americas, Europe, the Middle East, and North Africa (corresponding to WHO’s Eastern Mediterranean Region) and Asia (corresponding to WHO’s South East Asia and Western Pacific Regions).
Acknowledgements
This report was researched and written by Laura Thomas, researcher in the Health and Human Rights Division of Human Rights Watch. Additional research was conducted by Jane Crair, Operations Coordinator, and Alex Gertner, Associate in the Health and Human Rights Division at Human Rights Watch, and Emily Behar, Mari Milorava-Kelman, Michelle Persad, Myles Pulsford, Aliya Sanders, and Yumin Yang, all interns at Human Rights Watch. The report was edited by Diederik Lohman, senior researcher in the Health and Human Rights Division; Joseph Amon, director of the Health and Human Rights division; and Danielle Haas, senior editor at Human Rights Watch. It was reviewed by Rebecca Schleifer, advocacy director in the Health and Human Rights division; Clive Baldwin, senior legal advisor at Human Rights Watch; and Iain Levine, program director at Human Rights Watch. Relevant sections were reviewed by Maria Burnett, researcher in the Africa division at Human Rights Watch and Sarah Colm, senior researcher in the Asia Division at Human Rights Watch. Production assistance was provided by Alex Gertner, Anna Lopriore, creative manager; Grace Choi, publications director; Kathy Mills, publications coordinator and Fitzroy Hepkins, mail manager. Human Rights Watch is deeply grateful to the numerous healthcare workers who assisted us in preparing this report. All errors are our own.
Appendix 1 – List of Survey Participants
Please download a PDF of the report for this Appendix.
Appendix 2 – Survey Questions
Please download a PDF of the report for this Appendix.
Appendix 3 – Table of Calculations Used to Produce Maps
Please download a PDF of the report for this Appendix.
[1]J. Stjernsward & D. Clark, Palliative Medicine: A Global Perspective, in Derek Doyle et al., eds., Oxford Textbook of Palliative Medicine (Oxford: Oxford University Press, 3rd ed., 2003) pp. 1199-1222.
[2] “Briefing Note: Access to Controlled Medicines Program,” World Health Organisation Briefing Note, February 2009, http://www.who.int/medicines/areas/quality_safety/ACMP_BrNoteGenrl_EN_Feb09.pdf (accessed August 6, 2010).
[3]In the United States, most, but not all states, allow nurses to prescribe morphine. In Cameroon nurses with training in palliative care prescribe morphine, but it is not clear whether the law authorizes this.
[4]WHO, “National Cancer Control Programmes: Policies and Managerial Guidelines, second edition,” pp. 86-87.
[5] World Health Organization, “National Cancer Control Programs: Policies and Managerial Guidelines,” 2002, http://www.who.int/cancer/media/en/408.pdf (accessed August 6, 2010) pp. 85-86.
[6] Ibid., pp. 85, 91.
[7] Pain is also a symptom in various other diseases and chronic conditions and acute pain is often a side effect of medical procedures. This paper, however, focuses primarily on chronic pain.
[8] M. van den Beuken-van Everdingen et al., “Prevalence of Pain in Patients with Cancer: A Systematic Review of the Past 40 Years,” Annals of Oncology,vol. 18, no. 9 (2007) pp. 1437-1499.
[9]Charles S. Cleeland et al., “Multidimensional Measurement of Cancer Pain: Comparisons of U.S. and Vietnamese Patients,” Journal of Pain and Symptom Management vol. 3, no. 1 (1988); Charles S. Cleeland et al., “Dimensions of the Impact of Cancer Pain in a Four Country Sample: New Information from Multidimensional Scaling,”Pain, vol. 67, no. 2-3 (1996) pp. 267-73; Randall L. Daut & Charles S. Cleeland, “The Prevalence and Severity of Pain in Cancer,” Cancer vol. 50, no. 9 (1982) p. 1913; Kathleen M. Foley, Pain Syndromes in Patients with Cancer, in Kathleen M. Foley et al., eds., Advances in Pain Research and Therapy (1979) pp. 59-75;Kathleen M. Foley, “Pain Assessment and Cancer Pain Syndromes,” in Derek Doyle, Geoffrey W.C. Hanks and Neil MacDonald, Oxford Textbook of Palliative Medicine (Oxford: Oxford University Press, 2nd ed., 1999), pp. 310-331; J. Stjernsward & D. Clark, Palliative Medicine: A Global Perspective, in Derek Doyle et al., eds., Oxford Textbook of Palliative Medicine (Oxford: Oxford University Press, 3rd ed., 2003) pp. 1199-1222.
[10]K. Green, Evaluating the Delivery of HIV Palliative Care Services in Out-Patient Clinics in Viet Nam, Upgrading Document, London School of Hygiene and Tropical Medicine (2008); Kathleen M. Foley et al., “Pain Control for People with Cancer and AIDS,” in Dean T Jamison et al., Disease Control Priorities in Developing Countries (Washington: World Bank Publications, 2nd ed. 2003), pp. 981-994; Francois Larue et al., “Underestimation and Under-Treatment of Pain in HIV Disease: A Multicentre Study,” British Medical Journal, vol. 314 (1997) http://www.bmj.com/cgi/content/full/314/7073/23 (accessed August 6, 2010) p. 23; J. Schofferman & R. Brody, Pain in Far Advanced AIDS, in K. M. Foley et al., eds., Advances in Pain Research and Therapy (1990) pp. 379-386; E. J. Singer et al., “Painful Symptoms Reported by Ambulatory HIV-Infected Men in a Longitudinal Study," Pain, vol. 54 (1993) pp. 15-19.
[11] P. Selwyn & M. Forstein, “Overcoming the False Dichotomy of Curative vs. Palliative Care for Late-Stage HIV/AIDS,” Journal of the American Medical Association, vol. 290 (2003) pp. 806-814.
[12] M. C. Dalakas, “Peripheral Neuropathy and Antiretroviral Drugs”, Journal of the Peripheral Nervous System, vol. 6, no. 1 (2001) pp. 14-20; Several studies have found that between 29 and 74 percent of people who receive antiretroviral treatment experience pain symptoms: K. Green, Evaluating the Delivery of HIV Palliative Care Services in Out-Patient Clinics in Viet Nam, Upgrading Document, London School of Hygiene and Tropical Medicine (2008).
[13] F. Brennan, D.B. Carr and M. Cousins, “Pain Management: A Fundamental Human Right,” Anesthesia & Analgesia, vol. 105 (2007) pp. 205-221.
[14] O. Gureje et al., “Persistent Pain and Well-Being: A World Health Organization Study in Primary Care”, Journal of the American Medical Association, vol. 280(1998) pp. 147-51. See also: B. Rosenfeld et al., “Pain in Ambulatory AIDS Patients. II: Impact of Pain on Psychological Functioning and Quality of Life,"Pain, vol. 68, no. 2-3 (1996) pp. 323–28.
[15]B. Rosenfeld et al., “Pain in Ambulatory AIDS Patients. II: Impact of Pain on Psychological Functioning and Quality of Life,”Pain, pp. 323 – 28.
[16] R. L. Daut et al., “Developmentof the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases,” Pain, vol. 17, no. 2 (1983) pp. 197 – 210.
[17] World Health Organization, “Achieving Balance in National Opioid Control Policy,” 2000, http://apps.who.int/medicinedocs/en/d/Jwhozip39e/ (accessed August 6, 2010).
[18] World Health Organization, “WHO’s Pain Ladder,” 2010, http://www.who.int/cancer/palliative/painladder/en/ (accessed August 6, 2010). This has been developed for cancer but is also referred to for other conditions.
[19] World Health Organization, “Model List of Essential Medicines - 16th List,” March 2009, http://www.who.int/selection_medicines/committees/expert/17/sixteenth_adult_list_en.pdf (accessed 6 August 2010).
[20] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability 14 (2nd ed. 1996).
[21] Jennifer S. Temel, M.D., Joseph A. Greer, Ph.D., Alona Muzikansky, M.A., Emily R. Gallagher, R.N., Sonal Admane, M.B., B.S., M.P.H., Vicki A. Jackson, M.D., M.P.H., Constance M. Dahlin, A.P.N., Craig D. Blinderman, M.D., Juliet Jacobsen, M.D., William F. Pirl, M.D., M.P.H., J. Andrew Billings, M.D., and Thomas J. Lynch, M.D., Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer N Engl J Med 2010; 363:733-742, August 19, 2010
[22]“Briefing Note: Access to Controlled Medicines Program,” World Health Organisation Briefing Note, February 2009, http://www.who.int/medicines/areas/quality_safety/ACMP_BrNoteGenrl_EN_Feb09.pdf (accessed August 6, 2010);Sevil Atasoy, Statement by Professor Sevil Atasoy President of the International Narcotics Control Board (2009) http://www.incb.org/documents/President_statements_09/2009_ECOSOC_Substantive_Session_published.pdf p. 2; World Health Organization, “Model List of Essential Medicines - 16th List,” March 2009, http://www.who.int/selection_medicines/committees/expert/17/sixteenth_adult_list_en.pdf (accessed August 6, 2010).
[23]Michael Wright and others, “Mapping levels of palliative care development: a global view,” International Observatory on End of Life Care, Lancaster University, November 2006, www.eolc-observatory.net/global/pdr/world_map.pdf (accessed February 10, 2011).
[24] Human Rights Watch, “Please, do not make us suffer any more…”:Access to Pain Treatment as Human Right, March 2009, http://www.hrw.org/sites/default/files/reports/health0309web_1.pdf.
[25] Human Rights Watch, “Please, do not make us suffer any more…”: Access to Pain Treatment as Human Right, March 2009, http://www.hrw.org/sites/default/files/reports/health0309web_1.pdf. See Appendix 2 for survey questions.
[26] “Briefing Note: Access to Controlled Medicines Program,” World Health Organisation Briefing Note, February 2009, http://www.who.int/medicines/areas/quality_safety/ACMP_BrNoteGenrl_EN_Feb09.pdf (accessed August 6, 2010).
[27]World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability, (2nd ed. 1996).The need for a comprehensive palliative care policies is also stressed by academic experts: Stjernsward, J. & D. Clark, Palliative Medicine: A Global Perspective, in Derek Doyle et al., eds., Oxford Textbook of Palliative Medicine (Oxford: Oxford University Press, 3rd ed. 2003) pp. 1199-1222; DFID Health Resource Center, Review of Global Policy Architecture and Country Level Practice on HIV/AIDS and Palliative Care (2007).
[28] UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915.
[29]Human Rights Watch email correspondence with Dr Roberto Wenk, Argentina, October 18, 2010; Human Rights Watch email correspondence with Dr Roberto Bettega, Brazil, December 10, 2010.
[30]Human Rights Watch interviews with Indonesian doctors who requested anonymity, January 19, 2010, and November 5, 2010.
[31] Human Rights Watch, Unbearable Pain: India’s Obligation to Ensure Palliative Care, October 2009, http://www.hrw.org/en/reports/2009/10/28/unbearable-pain-0.
[32]“High-burden” is defined as adult HIV prevalence greater than 5%; data from UNAIDS, Report on the Global AIDS Epidemic, 2010, http://www.unaids.org/globalreport/ (accessed March 11, 2011).
[33] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability, (2nd ed. 1996); World Health Organization, “National Cancer Control Programs: Policies and Managerial Guidelines,” 2002, http://www.who.int/cancer/media/en/408.pdf (accessed August 6, 2010), pp. 86.
[34]K. Green, Evaluating the Delivery of HIV Palliative Care Services in Out-Patient Clinics in Viet Nam, Upgrading Document, London School of Hygiene and Tropical Medicine (2008); Kathleen M. Foley et al., “Pain Control for People with Cancer and AIDS,” in Dean T Jamison et al., Disease Control Priorities in Developing Countries (Washington: World Bank Publications, 2nd ed. 2003), pp. 981-994; Francois Larue et al., “Underestimation and Under-Treatment of Pain in HIV Disease: A Multicentre Study,” British Medical Journal, vol. 314 (1997) http://www.bmj.com/cgi/content/full/314/7073/23 (accessed August 6, 2010) p. 23; J. Schofferman & R. Brody, Pain in Far Advanced AIDS, in K. M. Foley et al., eds., Advances in Pain Research and Therapy (1990) pp. 379-386; E. J. Singer et al., “Painful Symptoms Reported by Ambulatory HIV-Infected Men in a Longitudinal Study," Pain, vol. 54 (1993) pp. 15-19; P. Selwyn & M. Forstein, “Overcoming the False Dichotomy of Curative vs. Palliative Care for Late-Stage HIV/AIDS,” Journal of the American Medical Association, vol. 290 (2003) pp. 806-814; Richard Harding et al., “Does Palliative Care Improve Outcomes for patients with HIV/AIDS: A systematic review of the evidence,” Sexually Transmitted Infections , vol. 81 (2005), pp. 5-14; Justin Amery et al., “The Beginning of Children’s Palliative Care in Africa: Evaluation of a Children’s Palliative Care Service in Africa,” Journal of Palliative Medicine, vol. 12 (2009), pp. 1015-1021.
[35] World Health Organization, “Model List of Essential Medicines - 16th List,” March 2009, http://www.who.int/selection_medicines/committees/expert/17/sixteenth_adult_list_en.pdf (accessed August 6, 2010), includes the following opioid analgesics: Codeine Tablet: 30 mg (phosphate); Morphine Injection: 10 mg (morphine hydrochloride or morphine sulfate) in 1‐ml ampoule; Oral liquid: 10 mg (morphine hydrochloride or morphine sulfate)/5 ml., Tablet: 10 mg (morphine sulfate); Tablet (prolonged release): 10 mg; 30 mg; 60 mg (morphine sulfate).
[36] Such fears were mentioned by healthcare workers from Bangladesh, Brazil, Cambodia, Cameroon, China, Colombia, the Dominican Republic, Ethiopia, Guatemala, Nepal, Philippines, South Africa, South Korea, Tanzania, Thailand, and Vietnam.
[37] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability (2nd ed. 1996).
[38]See Chapter IX for more detail on governments’ obligation to ensure that healthcare workers receive education in palliative care.
[39] Human Rights Watch interview with Professor Lucas Radbruch, Germany, February 4, 2010.
[40] Single Convention on Narcotic Drugs, United Nations, Single Convention on Narcotic Drugs (1961) http://www.incb.org/pdf/e/conv/convention_1961_en.pdf (accessed August 6, 2010).
[41] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability (2nd ed. 1996).
[42] Single Convention on Narcotic Drugs, United Nations, Single Convention on Narcotic Drugs (1961) http://www.incb.org/pdf/e/conv/convention_1961_en.pdf (accessed August 6, 2010), preamble.
[43]UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915 para. 43.
[44]For more discussion of this problem in one particular country, Kenya, see Human Rights Watch, Needless Pain: Government Failure to Provide Palliative Care for Children in Kenya, September 2010, http://www.hrw.org/en/reports/2010/09/09/needless-pain-0.
[45]WHO, Cancer Pain Relief: with a guide to opioid availability, 2nd ed. (Geneva: World Health Organization, 1996),
http://whqlibdoc.who.int/publications/9241544821.pdf (accessed February 20, 2011), p. 49.
[46]Argentina, Bangladesh, Cambodia, Cameroon, China, Ecuador, Egypt, El Salvador, Ethiopia, Germany, Guatemala, India, Indonesia, Iran, Kenya, Nepal, Pakistan, Philippines, South Africa, South Korea, Thailand, the United Kingdom and the United States. In Germany, South Korea, the United Kingdom and the United States, survey respondents commented that as there is good availability of opioids and complete consumption data is available to the government, such consultations are probably unnecessary.
[47]France, Jordan, Morocco, Nigeria, Ukraine, and Vietnam.
[48] United Nations, Single Convention on Narcotic Drugs (1961). http://www.incb.org/incb/convention_1961.html (accessed August 6, 2010).
[49]WHO, Cancer Pain Relief: with a guide to opioid availability, 2nd ed. (Geneva: World Health Organization, 1996),
http://whqlibdoc.who.int/publications/9241544821.pdf (accessed February 20, 2011), p. 56.
[50] Single Convention on Narcotic Drugs, United Nations, Single Convention on Narcotic Drugs (1961) http://www.incb.org/pdf/e/conv/convention_1961_en.pdf (accessed August 6, 2010), preamble;UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915
005090be?Opendocument (accessed January 17, 2010), para. 43; WHO, Cancer Pain Relief: with a guide to opioid availability, 2nd ed. (Geneva: World Health Organization, 1996),
http://whqlibdoc.who.int/publications/9241544821.pdf (accessed February 20, 2011), p. 56; Human Rights Watch, “Please, do not make us suffer any more…”: Access to Pain Treatment as Human Right, March 2009, http://www.hrw.org/sites/default/files/reports/health0309web_1.pdf.
[51] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability 10 (2nd ed. 1996).
[52]See Chapter VII for more detail on governments’ obligation to make pain medicines available under the right to the highest available standard of health.
[53] Human Rights Watch interview with Don Schumacher, president and CEO, National Hospice and Palliative Care Association, United States of America, February 8, 2010.
[54] Human Rights Watch interview with Dr Francis Javier, Director, Pain Management Centre, Philippines, August 26, 2009.
[55] Ibid.; Human Rights Watch interviews with Professor Mhamed Harif and Dr Maati Nejmi, Morocco, January 21, 2010.
[56] Human Rights Watch email correspondence with an Egyptian pain management specialist who requested anonymity, Egypt, October 7, 2010; Nathan I. Cherney et al., “Formulary Availability and Regulatory Barriers to Accessibility of Opioids for Cancer Pain in Europe: A Report from the ESMO/EAPC Opioid Policy Initiative,” Annals of Oncology, vol. 21, no. 3 (2010).
[57]Human Rights Watch email correspondence with a Russian doctor who requested anonymity, December 12, 2010.
[58]Human Rights Watch interview with Don Schumacher, February 8, 2010; Human Rights Watch interview with Dr. Bill Noble, Macmillan Senior Lecturer in Palliative Medicine, Sheffield University, United Kingdom, December 14, 2009; Human Rights Watch interview with Dr Liz Gwyther, CEO, Hospice Palliative Care Association of South Africa, South Africa, October 6, 2010; Human Rights Watch interview with Dr Henry Ddungu, advocacy manager, African Palliative Care Association, Uganda, August 10, 2009.
[59] J. Jagwe and A. Merriman, “Uganda: Delivering Analgesia in Rural Africa: Opioid Availability and Nurse-prescribing,” Journal of Pain and Symptom Management, vol. 33 no. 5 (2007), p. 547.
[60] International Narcotics Control Board, “Report of the International Narcotics Control Board for 2004,” E.05.XI.3, 2005, http://www.incb.org/pdf/e/ar/2004/incb_report_2004_full.pdf (accessed October 28, 2010), para. 196.
[61] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability 10 (2nd ed. 1996).
[62] Human Rights Watch interview with Dr Maati Nejmi, January 21, 2010; Human Rights Watch interviews with Professor Lucas Radbruch, Palliative Medicine, Aachen University, Germany, February 4, 2010, Professor Rolf-Detlef Treede, president of the German Pain Society, Germany, October 12, 2009, and Dr Henry Lu, immediate past president of the Pain Society of the Philippines, Philippines, September 2, 2009.
[63]Human Rights Watch interview with Professor Serdar Erdine, chairman, Department of Pain Management, Istanbul University, president of the Turkish Society of Pain Management, Turkey, November 19, 2009; Human Rights Watch Interviews with Dr Larin Lovo and Dr Carlos Eduardo Rivas, El Salvador, 2010; Human Rights Watch interview with Viktoria Tymoshevska, International Palliative Care Initiative, Ukraine, September 24, 2010; Human Rights Watch interview Nathan I. Cherney et al., “Formulary Availability and Regulatory Barriers to Accessibility of Opioids for Cancer Pain in Europe: A Report from the ESMO/EAPC Opioid Policy Initiative,” Annals of Oncology, vol. 21, no. 3 (2010), p. 620.
[64] The Ministry of Health of Ukraine: Order No. 356; Human Rights Watch interview with Victoria Tymosnevska, Ukraine, September 24, 2010.
[65] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability 10-11 (2nd ed. 1996).
[66]See Chapter VIII for more detail on governments’ obligation to make pain medicines available under the right to the highest available standard of health.
[67]Human Rights Watch interviews with doctors in China, February, October, and November, 2010.
[68] International Narcotics Control Board, Demandfor and Supply of Opiates for Medical and Scientific Needs, 15 (1989).
[69]See Chapter VII for more detail on governments’ obligation under the right to health to ensure that regulation of morphine prescribing does not unreasonably make pain medicines unavailable under the right to the highest available standard of health.
[70]Scott Burris & Corey S. Davis, A Blueprint for Reforming Access to Therapeutic Opioids: Entry Points for International Action to Remove the Policy Barriers to Care, Centers for Law and the Public's Health: A Collaborative at Johns Hopkins and Georgetown Universities, 2008, http://www.painpolicy.wisc.edu/internat/DCAM/Burris_Blueprint_for_Reform.pdf, (accessed November 2, 2010) p. 18; In low and middle-income countries a typical daily dose of morphine for patients in palliative care programs is 60 to 75 milligrams per day: Kathleen M. Foley et al., “Pain Control for People with Cancer and AIDS,” in Dean T Jamison et al., Disease Control Priorities in Developing Countries (Washington: World Bank Publications, 2nd ed. 2003), pp. 981-994. The average daily dose in industrialized countries tends to be higher. This is due, among other reasons, to longer survival of patients and the development among patients of tolerance to opioid analgesics—based on Human Rights Watch e-mail correspondence with Dr. Kathleen M. Foley, January 23, 2009.
[71] Liliana De Lima et al., “Potent Analgesics Are More Expensive for Patients in Developing Countries: A Comparative Study,” Journal of Pain and Palliative Care Pharmacotherapy, vol. 18, no. 1, (2004), p. 63.
[72] Ibid; David E. Joransen, M.R. Rajagopal and Aaron M. Gilson, “Improving Access to Opioid Analgesics for Palliative Care in India,” Journal of Pain and Symptom Management, vol. 24, no. 2 (2002), pp. 152-59.
[73] Liliana De Lima et al., “Potent Analgesics Are More Expensive for Patients in Developing Countries: A Comparative Study,” Journal of Pain and Palliative Care Pharmacotherapy, vol. 18, no. 1, (2004), p. 63.
[74] International Association for Hospice and Palliative Care, IAHPC List of Essential Medicines for Palliative Care, undated, http://www.hospicecare.com/resources/pdf-docs/iahpc-essential-meds-en.pdf (accessed November 2, 2010).
[75]UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915
005090be?Opendocument (accessed January 17, 2010), para. 43.
[76]See Chapter VIII for more detail on governments’ obligation to make pain medicines available under the right to the highest available standard of health.
[77] World Health Organization, “National Cancer Control Programs: Policies and Managerial Guidelines,” 2002, http://www.who.int/cancer/media/en/408.pdf (accessed August 6, 2010), pp. 85, 91.
[78] World Health Organization, Cancer Pain Relief: a Guide To Opioid Availability, (2nd ed. 1996); World Health Organization, “National Cancer Control Programs: Policies and Managerial Guidelines,” 2002, http://www.who.int/cancer/media/en/408.pdf (accessed August 6, 2010), pp. 86.
[79]UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915
005090be?Opendocument (accessed January 17, 2010), paras. 18-19, 43.
[80] Supply Chain Management Solutions, http://scms.pfscm.org/scms (accessed December 1, 2010); IDA Foundation, http://www.idafoundation.org/we-are.html (accessed December 1, 2010); Health Action International, http://www.haiweb.org/ (accessed December 1, 2010).
[81]Human Rights Watch interview with head nurse, Bondo District Hospital, Kenya, March 1, 2010.
[82] Note that we asked what facilities usuallystock morphine. Stock-outs are common in many African countries. In 2010, Kenya and Uganda both faced interruptions of morphine supply.
[83]Email correspondence with Anne Merriman, director of policy and international programs, Hospice Africa Uganda, June 22, 2010.
[84]Proclamation No. 176/1999 a Proclamation to provide for Drug Administration and Control, s 26(1) available at United Nations Office of Drugs and Crime Legal Library: http://www.unodc.org/enl/showDocument.do?documentUid=2720&country=ETH (accessed January 13, 2011).
[85] International Narcotics Control Board, “Narcotic Drugs: Estimated World Requirements for
2010: Statistics for 2008,” 2010, http://www.incb.org/incb/en/narcotic_drugs_reports.html (accessed 27 October 2010).
[86] Jagwe and Merriman, “Uganda: Delivering Analgesia in Rural Africa,” Journal of Pain and Symptom Management.
[87] INCB, Report of the International Narcotics Control Board for 2004 (New York: United Nations, 2005), pp. 32-33.
[88] Palliative Care Association of Uganda (PCAU), “Audit Report of Palliative Care Services in Uganda,” April 2009, http://www.theworkcontinues.com/document.asp?id=1386&pageno= (accessed March 27, 2010), pp. 7 and 12.
[89] Jack Jagwe and Anne Merriman, “Uganda: Delivering Analgesia in Rural Africa: Opioid Availability and Nurse-prescribing,” Journal of Pain and Symptom Management, vol. 33, no. 5 (May 2007); Stjernsward, “Uganda: Initiating a Government Public Health Approach to Pain Relief and Palliative Care,” Journal of Pain and Symptom Management.
[90]Jan Stjernsward, “Uganda: Initiating a Government Public Health Approach to Pain Relief and Palliative Care,” Journal of Pain and Symptom Management, vol. 24, no. 2 (August 2002).
[91] PCAU, “Audit Report of Palliative Care Services in Uganda,” p. 8.
[92]Human Rights Watch email correspondence with Dr Roberto Wenk, Argentina, October 18, 2010; Human Rights Watch email correspondence with Dr Roberto Bettega, Brazil, December 10, 2010.
[93]Colombian Minister of Health 001478 Resolution of 2006, https://www.alcaldiabogota.gov.co/sisjur/normas/normal.jsp.old (accessed January 27, 2011).
[94] Leon, Marta et al. “Integrating palliative care in public health: The Colombian experience following an International Pain Policy Fellowship” http://www.painpolicy.wisc.edu/publicat/11pallmed/LeonIPPF2011.pdf (accessed April 24, 2011).
[95] Ibid.
[96] Ibid.
[97] Ibid.
[98] Leon, Marta Ximena et al. Improving Availability of and Access to Opioids in Colombia: Description and Preliminary Results of an Action Plan for the Country.
[99] Ibid.
[100] Ibid.
[101] Congresso Visible, http://www.congresovisible.org/proyectos-de-ley/mediante-la-cual-se-regulan/1080/ (accessed April 1, 2011).
[102]A further three countries (the United States, the United Kingdom, and Germany) reported that they do not have an essential medicine list, and there were conflicting responses from survey respondents in Mexico.
[103]“Implementation of Comprehensive National Cancer Control Program in Iran: an experience in a developing country” Annals of Oncology, Vol. 19, No.2, February 2008. p. 399.
[104] Jan Stjernswärd et al., “Jordan Palliative Care Initiative: A WHO Demonstration Project,” Journal of Pain and Symptom Management, vol. 33, no. 5 (2007), p. 631.
[105] Amanda Bingley and David Clark, “A Comparative Review of Palliative Care Development in Six Countries Represented by the Middle East Cancer Consortium (MECC),” Journal of Pain and Symptom Management, vol. 37, no. 3 (2009) p. 291.
[106] Jan Stjernswärd et al., “Jordan Palliative Care Initiative: A WHO Demonstration Project,” Journal of Pain and Symptom Management, vol. 33, no. 5 (2007), p. 629.
[107] Ibid., p. 630
[108] Ibid.
[109] Kathleen M. Foley et al., “Pain Control for People with Cancer and AIDS,” in Dean T Jamison et al., Disease Control Priorities in Developing Countries (Washington: World Bank Publications, 2nd ed. 2003), pp. 981-994; Germany imposes a limit of 20,000mg per prescription, but this can be exceeded if the doctor marks the prescription in a specified manner.
[110] Article 54 of the regulation.
[111] Daniela Mosoiu et al., “Romania: Changing the Regulatory Environment”, Journal of Pain and Symptom Management, vol. 33, no. 5 (2007), p. 613.
[112] Human Rights Watch interview with Associate Professor Dr. Daniela Mosoiu, Romania, February 17, 2010.
[113] Consumption Data. International Narcotics Control Board.
[114] While this is an improvement, patients and their families can only fill prescriptions for 10 days at a time, after which their local commune must confirm in writing that the patient is still alive.
[115] Human Rights Watch interview with Dr Eric Krakauer, November 3, 2009.
[116]Eric L. Krakauer, Nguyen Thi Phuong Cham, Luong Ngoc Khue. Vietnam's Palliative Care Initiative: Successes and Challenges in the First Five Years. Journal of Pain and Symptom Management, 2010 40(1): 27-30.
[117]Ibid.
[118] Human Rights Watch interview with Dr Eric Krakauer, November 3, 2009.
[119]Green K, Kinh LN, Khue LN., “Palliative care in Vietnam: Findings from a rapid situation analysis in five provinces,” (Hanoi: Vietnam Ministry of Health, 2006).
[120] Human Rights Watch interview with Dr. Eric Krakauer, November 3, 2009.
[121]International Covenant on Economic, Social and Cultural Rights (ICESCR), G.A. res. 2200A (XXI), 21 U.N.GAOR Supp. (No. 16) at 49, U.N. Doc. A/6316 (1966), 993 U.N.T.S. 3, entered into force January 3, 1976, art. 11; See also Convention on the Rights of the Child, G.A. res. 44/25, annex, 44 U.N. GAOR Supp. (No. 49) at 167, U.N. Doc. A/44/49 (1989), entered into force September 2 1990, art. 24.
[122]International Covenant on Economic, Social and Cultural Rights (ICESCR), G.A. res. 2200A (XXI), 21 U.N.GAOR Supp. (No. 16) at 49, U.N. Doc. A/6316 (1966), 993 U.N.T.S. 3, entered into force January 3, 1976, art. 12.
[123]The Right to the Highest Attainable Standard of Health, Committee on Economic, Social and Cultural Rights, E/C.12/2000/4. (General Comments), 11 August 2000, I.12.(a).
[124]UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000),http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915005090be?Opendocument (accessed November 4, 2010), [hereinafter Substantive Issues Arising in the Implementation of the ICESCR, General Comment No. 14], paras. 30 and 33.
[125] Ibid., para. 25.
[126] Ibid., para. 19.
[127] Ibid., para. 25. While the committee included this reference in a paragraph on the right to health for older persons, the wording clearly indicates that it applies to all chronically and terminally ill persons.
[128] The Right to the Highest Attainable Standard of Health, Committee on Economic, Social and Cultural Rights, E/C.12/2000/4. (General Comments), 11 August 2000, III.47.
[129] Ibid., para. 43.
[130] Ibid., para. 44.
[131] Ibid., para. 38.
[132] World Health Organization, “Model List of Essential Medicines - 16th List,” March 2009, http://www.who.int/selection_medicines/committees/expert/17/sixteenth_adult_list_en.pdf (accessed 6 August 2010), includes the following opioid analgesics: Codeine Tablet: 30 mg (phosphate); Morphine Injection: 10 mg (morphine hydrochloride or morphine sulfate) in 1‐ml ampoule; Oral liquid: 10 mg (morphine hydrochloride or morphine sulfate)/5 ml., Tablet: 10 mg (morphine sulfate); Tablet (prolonged release): 10 mg; 30 mg; 60 mg (morphine sulfate).
[133]Substantive Issues Arising in the Implementation of the ICESCR, General Comment No. 14, para. 12.
[134] Ibid.
[135] Ibid.
[136] World Health Organization, “National Cancer Control Programs: Policies and Managerial Guidelines,” 2002, http://www.who.int/cancer/media/en/408.pdf (accessed August 6, 2010) pp. 86.
[137] International Covenant on Civil and Political Rights (ICCPR), adopted December 16, 1966, G.A. Res. 2200A (XXI), 21 U.N. GAOR Supp. (No. 16) at 52, U.N. Doc. A/6316 (1966), 999 U.N.T.S. 171, entered into force March 23, 1976, art. 7 provides, “No one shall be subjected to torture or to cruel, inhuman or degrading treatment or punishment”; Universal Declaration of Human Rights (UDHR), adopted December 10, 1948, G.A. Res. 217A(III), U.N. Doc. A/810 at 71 (1948); Convention against Torture and Other Cruel, Inhuman or Degrading Treatment or Punishment (Convention against Torture), adopted December 10, 1984, G.A. res. 39/46, annex, 39 U.N. GAOR Supp. (No. 51) at 197, U.N. Doc. A/39/51 (1984), entered into force June 26, 1987, article 16 provides that “Each State Party shall undertake to prevent in any territory under its jurisdiction other acts of cruel, inhuman or degrading treatment or punishment which do not amount to torture as defined in article I, when such acts are committed by or at the instigation of or with the consent or acquiescence of a public official or other person acting in an official capacity”; Inter-American Convention to Prevent and Punish Torture, O.A.S. Treaty Series No. 67, entered into force February 28, 1987; European Convention for the Prevention of Torture and Inhuman or Degrading Treatment or Punishment (ECPT), signed November 26, 1987, E.T.S. 126, entered into force February 1, 1989; African [Banjul] Charter on Human and Peoples' Rights, adopted June 27, 1981, OAU Doc. CAB/LEG/67/3 rev. 5, 21 I.L.M. 58 (1982), entered into force October 21, 1986.
[138] See for example the judgment of the European Court of Rights in Z v United Kingdom (2001) 34 EHRR 97.
[139]Joint letter by the UN special rapporteur on the prevention of torture and cruel, inhuman or degrading treatment or punishment, Manfred Nowak, and the UN special rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health, Anand Grover, to the Commission on Narcotic Drugs, December 2008. A copy of the letter is available at http://www.ihra.net/Assets/1384/1/SpecialRapporteursLettertoCND012009.pdf (accessed April 27, 2010).
[140]Human Rights Council, Report of the Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment, Manfred Nowak, U.N. Doc. A/HRC/10/44, January 14, 2009, paras. 72 and 74(e).
[141]This method was adapted from the methodology presented in Seya et al., “A First Comparison Between the Consumption of and the Need for Opioid Analgesics at Country, Regional, and Global Levels,” Journal of Pain & Palliative Care Pharmacotherapy, vol. 25, (2001), p. 6.
[142]International Narcotics Control Board, “Narcotic Drugs: Estimated World Requirements for
2010: Statistics for 2008,” 2010, http://www.incb.org/incb/en/narcotic_drugs_reports.html (accessed 27 October 2010) pp. 208 – 258.
[143] Kathleen M. Foley et al., “Pain Control for People with Cancer and AIDS,” in Dean T. Jamison et al., eds., Disease Control Priorities in Developing Countries, 2nd ed. (New York: Oxford University Press, 2006), p. 982.
[144] World Health Organisation, “Global Health Observatory,” 2009, http://apps.who.int/ghodata/ (accessed 28 October 2010).