Uncontrolled Pain
Ukraine’s Obligation to Ensure Evidence-Based Palliative Care
Map of Ukraine
Glossary
Ambulatoria: An outpatient clinic that together with the feldshersko-akusherski punkt (FAP) is often the only source of healthcare available to patients in rural areas.
Analgesic: A medicine that reduces pain.
Central District Hospital: The main health facility and administrative center for the public healthcare system. Each of Ukraine’s 490 districts has one.
Chronic pain: Defined in this report as pain that occurs over weeks, months, or years rather than a few hours or days. Because of its duration, moderate to severe chronic pain should be treated with oral opioids rather than repeated injections, especially for people emaciated by diseases such as cancer and HIV/AIDS.
Controlled medicines: Medicines that contain controlled substances.
Controlled substances: Substances that are listed in one of the three international drug control conventions: the Single Convention on Narcotic Drugs of 1961 as amended by the 1972 Protocol; the Convention on Psychotropic Substances of 1971; and the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, 1988.
Dependence: Defined by the World Health Organization (WHO) Expert Committee on Drug Dependence as a “cluster of physiological, behavioral and cognitive phenomena of variable intensity, in which the use of a psychoactive drug (or drugs) takes on a high priority. The necessary descriptive characteristics are preoccupation with a desire to obtain and take the drug and persistent drug-seeking behavior. Determinants and problematic consequences of drug dependence may be biological, psychological or social, and usually interact.”[1] Dependence is clearly established to be a disorder. For Dependence syndrome, WHO’s International classification of diseases, 10th Edition (ICD-10), requires that three or more of the following six characteristic features have been experienced or exhibited:
- A strong desire or sense of compulsion to take the substance;
- Difficulty controlling the onset, termination, and levels of use of substance-taking behavior;
- Physiological withdrawal state when substance use has ceased or been reduced, as evidenced by: the characteristic withdrawal syndrome for the substance; or use of the same (or a closely related) substance with the intention of relieving or avoiding withdrawal symptoms;
- Evidence of tolerance, such that increased doses of the psychoactive substance are required in order to achieve effects originally produced by lower doses;
- Progressive neglect of alternative pleasures or interests because of psychoactive substance use, increased amount of time necessary to obtain or take the substance or to recover from its effects;
- Persisting with substance use despite clear evidence of overtly harmful consequences, such as harm to the liver through excessive drinking, depressive mood states after periods of heavy substance use, or drug-related impairment of cognitive functioning; efforts should be made to determine that the user was actually, or could be expected to be, aware of the nature and extent of the harm.
The Expert Committee on Drug Dependence (ECDD) concluded “there were no substantial inconsistencies between the definitions of dependence by the ECDD and the definition of dependence syndrome by the ICD-10.”
Diversion: The movement of controlled drugs from licit to illicit distribution channels or to illicit use.
Essential medicines: Those medicines that are listed on the WHO Model List of Essential Medicines or the WHO Model List of Essential Medicines for Children. Both model lists present a list of minimum medicine needs for a basic healthcare system, listing the most efficacious, safe, and cost-effective medicines for priority conditions.
Feldshersko-akusherski punkt(FAP): A local health clinic that provides basic procedures, including prenatal care and first aid. These health centers are run by feldshers, physician assistants trained in vocational medical schools. They provide routine checkups, immunizations, emergency first-aid, and midwifery services. There are no physicians at these clinics.
Hospice: A specialist palliative care facility. In Ukraine, hospices are exclusively in-patient facilities.
Life-limiting illness: A broad range of conditions including cancer, HIV/AIDS, dementia, heart, renal, and liver disease, and permanent serious injury, in which painful or distressing symptoms occur; although there may also be periods of healthy activity, there is usually at least a possibility of premature death.
Misuse (of a controlled substance): Defined in this report as the non-medical and non-scientific use of substances controlled under the international drug control treaties or national law.
Morphine: A strong opioid medicine that is the cornerstone for treatment of moderate to severe cancer pain. The WHO considers morphine an essential medicine in its injectable, tablet, and oral solution formulations.
Narcotic drugs: A legal term that refers to all those substances listed in the Single Convention.
Opioid: The term means literally “opium-like substance.” It can be used in different contexts with different but overlapping meanings. In pharmacology, it refers to chemical substances that have similar pharmacological activity as morphine and codeine, i.e. analgesic properties. They can stem from the poppy plant, be synthetic, or even made by the body (endorphins).
Over-the-counter pain medicines: Non-opioid pain medicines suitable for mild pain, including paracetamol (also known as acetaminophen), aspirin, and ibuprofen.
Palliative care: Health care that aims to improve the quality of life of people facing life-limiting illness, through pain and symptom relief and psychosocial support for patients and their families. Palliative care can be delivered in parallel with curative treatment but its purpose is to care, not cure.
Psychosocial support: A broad range of services for patients and their families to address the social and psychological issues they face due to life-limiting illness. Psychologists, counselors, and social workers often provide these services.
Strong opioid analgesics: Pain medicines that contain strong opioids, such as morphine, methadone, fentanyl, and oxycodone and are used to treat moderate to severe pain.
Weak opioid analgesics: Pain medicines that are generally used to treat mild to moderate pain, including codeine, dihydrocodeine, and tramadol.
Prologue: The Story of Vlad Zhukovsky
Born in 1983, Vladislav (Vlad) was in many ways a young Ukrainian no different than many others. He lived with his mother and sister in a two-bedroom flat in a Soviet-style apartment park on the outskirts of Cherkassy in central Ukraine. He loved playing his guitar and taking walks along the River Dnepr. He faithfully attended church with a tight knit group of friends and had a knack for computers, a talent he hoped to turn into a career.
Vlad’s ordinary life abruptly ended in 2001 when he was a second-year student of computer technology. One day in class, he developed a headache that was so severe that, according to his mother, he fell down, crying in pain. “We gave him analgin [a commonly used pain medication] but nothing worked,” said Nadezhda (Nadya), Vlad’s mother. “He just grabbed his head and screamed.”[2] She called an ambulance to take Vlad to the hospital, where brain scans revealed a large medulloblastoma of the cerebellum, a malignant tumor.
A fierce battle with cancer ensued. Radiation initially forced the brain tumor into remission. But the cancer kept coming back. Over the next nine years, tumors formed in Vlad’s lower spine, again in his head, his chest, and eventually again his spine. With each new tumor, the periods of remission grew shorter and Vlad weaker.
Throughout this ordeal, Vlad and his mother fought a second battle: with pain. This battle, at least, should not have been a losing one. The World Health Organization (WHO) says that “[m]ost, if not all, pain due to cancer could be relieved if we implemented existing medical knowledge and treatments.”[3] But as Vlad learned, the way Ukraine’s healthcare system treats cancer pain has little in common with current medical knowledge.
In 2007 Vlad developed persistent, severe pain that over-the-counter pain medicines could no longer relieve. According to his mother, who devotedly took care of her son, the pain was so bad that he often screamed in agony, sometimes so loud that it disturbed their neighbors. She told Human Rights Watch: “Hearing his pain, how he struggled, how he howled, it was just impossible to be in the [same] room.” The pain deprived him of sleep, made him moody, disrupted normal interaction with family and friends, and—possibly worst of all for a young man who liked to be active—reduced him to passively lying in bed and staring at the ceiling. Indeed, the pain incapacitated Vlad more than his cancer.
While Vlad’s doctors did prescribe a strong pain medication to treat Vlad’s pain, they did so with inadequate regularity and insufficient doses to offer full relief. The WHO recommends that morphine or a pain medication of similar strength be given every four hours to ensure continuous relief and that “the ‘right’ dose is the dose that relieves the patient’s pain.”[4] Yet, Vlad’s doctors initially prescribed just three doses per day, leaving him without relief half of the time.
Nadya Zhukovski kisses her sleeping son Vlad before going to the local pharmacy for medical supplies. © Scott Anger & Bob Sacha for the Open Society Foundations.
One day, in June 2008, Vlad’s pain became so severe that he could no longer bear it and decided to jump from his hospital window. While his mother was pleading with nurses to give him more pain medications, Vlad climbed into the open window. Most of his body was already outside—his fall imminent—when his roommate, a retired police officer, noticed what was happening, grabbed him by the leg, and forced him back in. He later told his mother that he had wanted to fall “head down and be dead right away so it wouldn’t hurt anymore.” Vlad, who was very religious, was deeply troubled by his suicide attempt. He repeatedly told his mother afterwards that he worried that the pain might make him do something sinful that would prevent him from seeing her again in heaven.
No matter how obvious it was that Vlad’s pain was not under control, doctors met Nadya’s subsequent pleas for more pain medications with great reluctance, sometimes bordering on hostility. When she pleaded for a fourth dose, doctors at one hospital accused her of selling the medications on the street. A year later, as she tried to convince doctors at another hospital that her son needed a fifth dose, doctors claimed more of the medication would lead to an overdose and they would then face prosecution “like Michael Jackson’s doctor.”
Supported by his mother and a small, local nongovernmental palliative care organization called Face-to-Face, Vlad battled with the pain and the cancer. He tried to stay positive and enjoy those moments when he was not in pain. Even after the cancer invaded his spinal cord and paralyzed him from the waist down, his church friends would occasionally take him in a wheelchair to the River Dnepr for a short walk.
Vlad died in October 2010. A few months before his death, he said he hoped to be remembered as “an ordinary, happy person, as normal, sociable Vlad.”[5]
During this nine year ordeal, Vlad and his mother frequently spoke of the need for change in Ukraine’s healthcare system that caused him so much unnecessary suffering. Vlad did not want his agony to be in vain or suffered again by tens of thousands of Ukrainians battling cancer each year.
***
This report is dedicated to Vlad’s courage and memory, and to his mother Nadya.
Summary
Patients with life-limiting illnesses need curative treatment, but they also need palliative care, which aims to address pain and improve life quality diminished by debilitating symptoms such as shortness of breath, anxiety, and depression.[6]
Every year almost half a million people in Ukraine may require palliative care services to alleviate the symptoms of life-limiting illnesses.[7] These include circulatory system illnesses such as chronic heart disease (almost 489,000 deaths per year), cancer (100,000), respiratory illnesses (28,000), tuberculosis (10,000), neurological disorders such as Alzheimer’s disease (6,500), and HIV and AIDS (about 2,500).[8]
Relieving pain is a critical part of palliative care. About 80 percent of patients with advanced cancer develop moderate to severe pain, as do significant numbers of patients with HIV and other life-limiting illnesses. With existing medical knowledge physical pain can be successfully treated in most cases. But while these symptoms are treatable, limitations in Ukraine’s health policy, education, and drug availability; lack of cohesion, urgency, and coordination on the part of the government; unnecessarily onerous drug regulations; inadequate training and a dearth of exposure to palliative care services for Ukrainian healthcare providers mean the country’s public health system offers poor pain treatment and little support for families dealing with life-limiting illnesses.
The country has 9 hospices with a total of about 650 beds, which provide services to inpatients.[9] The government has also assigned palliative care beds in some other public hospitals, and the national cancer control plan envisions a total of 36 hospices by 2016, although it does not allocate a budget for this.[10] Despite this, most patients with life-limiting illnesses in Ukraine die at home; indeed, hospitals are not supposed to admit patients with cancer who are no longer receiving curative treatment. Yet there are no full-fledged home-based palliative care services.[11] Some nongovernmental organizations (NGOs) provide home-based care but cannot offer pain management with opioid analgesics, including morphine, which WHO guidelines for cancer pain emphasize, should be used to treat moderate to severe pain. Most AIDS centers do not offer palliative care services.[12] According to a 2011 International Narcotics Control Board (INCB) report, the amount of opioid analgesics Ukraine uses per year is “very inadequate.”[13]
In 2010 Human Rights Watch—together with the Institute of Legal Research and Strategies in Kharkiv and the Rivne and Kiev branches of the All-Ukrainian Network of People Living with HIV—researched the availability of pain treatment and palliative care in Ukraine. We found that Vlad’s unnecessary suffering was not an unfortunate anomaly. Rather, it was in many ways representative of the fate of patients who endure pain due to life-limiting diseases.
In dozens of interviews, patients, families, doctors, nurses, and government officials painted a picture of a healthcare system that systematically fails patients who are in severe pain because pain treatment is often inaccessible, best practices for palliative care are ignored, and anti-drug abuse regulations hamstring healthcare workers’ ability to deliver evidence-based care. Those healthcare workers who try to provide the most effective pain treatment possible must often operate, as one oncologist said, “on the edge of the law.”[14] These doctors and nurses ignore legal restrictions and provide patients with a take-home supply of strong pain medications or leave the day’s supply with patients to administer themselves. In doing so, these doctors and nurses expose themselves to administrative and criminal charges for putting patients’ well-being first.
The situation is particularly devastating in rural areas—home to about one-third of Ukraine’s population of 46 million—where strong opioid analgesics are often hard to access or simply unavailable.[15] Only central district hospitals have the necessary license to stock and dispense morphine and other strong opioid analgesics, according to doctors in rural districts who said requirements for obtaining such licenses are too onerous and costly for many smaller hospitals and health clinics.[16] As a result, people in rural towns and villages often live far from health centers with strong pain medications.
Distance might be surmountable if healthcare providers could give patients and their families a supply of strong opioid analgesics for at least a week or two. However, under Ukraine’s drug regulations healthcare workers must directly administer injectable strong opioid pain medications to patients, a requirement that is medically unnecessary. As oral morphine is unavailable in Ukraine, a nurse or other healthcare worker must travel to the patient’s home up to six times a day to administer pain medications (the WHO recommends that morphine is administered every four hours). This burden is too great for healthcare workers, leaving patients with severe pain in remote areas “doomed,” according to one nurse.[17]
Patients in urban areas face a different problem. Here, hospitals generally do have the license for strong opioid analgesics, but pain treatment is still often woefully inadequate, as healthcare workers routinely ignore the core principles for effective pain treatment that the World Health Organization has identified.[18] This leaves individuals with inadequate and inconsistent relief from excruciating pain.
There is no acceptable reason why Ukraine cannot deliver proper palliative care and pain management to patients with life-limiting illnesses. Although under-resourced, Ukraine has a healthcare system that is able to deliver effective treatment for various other health conditions.
Failure to address barriers to effective pain treatment identified in this report places Ukraine in violation of the right to health guaranteed by the International Covenant on Economic, Social and Cultural Rights (ICESCR), and in possible violation of the prohibition on torture and cruel, inhuman, or degrading treatment. It also ensures that Ukraine continues to remain out of step with its neighbors—including Belarus, Moldova, Russia, and Turkey–which have less restrictive drug regulations and with European countries that all (except for Armenia and Azerbaijan) have oral morphine available for patients.[19] Lack of action also means that Ukraine will continue to deviate fundamentally from World Health Organization recommendations in standard pain treatment practices, that healthcare workers will have to break the law to provide evidence-based care, and that patients will continue to suffer.
All medical students should receive basic instruction on palliative care and pain treatment. Those specializing in disciplines that frequently care for people with life-limiting illnesses should receive detailed instruction and exposure to clinical practice. Ukraine must urgently amend the restrictive and problematic licensing requirements for healthcare institutions and workers to stock, prescribe, or dispense opioid analgesics and must simplify the prescribing procedure that currently creates a barrier to timely treatment with morphine for patients with pain. Problematic dispensing procedures should be revised, and the current complex and wasteful record keeping system improved. Inspections of healthcare institutions that work with opioid analgesics should be conducted so as to minimize their impact on the provision of and access to medical care, and Ukraine’s criminal code should be amended to differentiate between intentional and unintentional violations of the rules of handling opioid medications.
***
Our research found that when strong opioid analgesics are available, they are provided in a way that fundamentally deviates from WHO recommendations, with each of the five core principles it has identified routinely ignored.[20]
Principle 1: Pain medications should be given orally whenever possible. If a patient cannot take medications by mouth, rectal suppositories, or under-the-skin injections should be used.In Ukraine no oral morphine is available. Doctors use only injectable strong opioids for pain treatment. Instead of injecting morphine under the skin, as the WHO recommends, injections are given into muscles. This results in large numbers of unnecessary intramuscular injections, which are unpleasant for patients and carry a risk of infection. Throughout the three years he was on strong pain medications, Vlad received thousands of unnecessary injections with pain medications. His mother compared his bottom, where most of the injections were administered, to a “mine field.”
Principle 2: Pain medications should be given every four hours to ensure continuous pain control.While the WHO recommends that patients receive strong pain medications every four hours, most patients in Ukraine get them only once or twice per day. As the effects of morphine last for four to six hours, this means that such patients are without adequate relief for most of the day. While doctors prescribe weaker pain medicines and other medications for the intervals, these are not potent enough to provide effective relief and expose patients to unnecessary side effects. Our research suggests this practice is largely due to the requirement in Ukrainian law that healthcare providers directly administer injectable strong opioid analgesics to patients. Doctors at various health facilities told us that they do not have the resources for a nurse to visit patients at home six times per day.
Principle 3: The type of pain medication (basic pain reliever, weak opioid, or strong opioid) should depend on severity of pain. If a pain medication stops providing effective relief, a stronger medication should be used. International research suggests that about 80 percent of terminal cancer patients need a strong opioid pain medicine for an average period of 90 days before death.[21] Yet figures we received from various hospitals in Ukraine about the percentage of cancer patients who receive morphine or other strong opioid analgesics and the average number of days patients receive them suggest that many patients are started on strong opioid analgesics late, if at all. In the six hospitals and one polyclinic department of a city hospital for which we received such data, we found that in the best case only about one-third of terminal cancer patients received a strong opioid analgesic—in most cases it was far less—and in some cases for far less than 90 days.
Principle 4: The dose of medication should be determined individually. There is no maximum dose for strong opioid pain medications. While the WHO treatment guideline specifically states there should be no maximum daily dose for morphine, both Ukraine’s Ministry of Health and the Zdorovye Narodu pharmaceutical company, the only manufacturer of morphine in Ukraine, recommend a maximum daily dose of 50 mg of injectable morphine. This dose is far below levels of morphine used safely and effectively for the treatment of severe pain in other countries. We found that many doctors in Ukraine, though not all, adhere to the recommendation and cap the dose even when the patient is still in pain.
Principle 5: Pain treatment should be delivered according to the patient’s needs. Because nurses have to come to patients’ homes to administer morphine injections, it is not the patient’s schedule but that of the healthcare worker that determines when the patient receives his medications. As a result, patients wait in agony for nurses to arrive or do not need the medicine when the nurse is present.
While our research focused mostly on the plight of cancer patients, we also documented a number of cases of people who had severe pain due to other diseases or health conditions. We found that these patients face even greater challenges in getting access to good pain treatment. General practitioners and other specialists are rarely trained in treating pain and often worry about prescribing strong opioid medications to non-cancer patients. Several patients with non-cancer pain told us that their doctors ignored their complaints about pain or told them it would simply go away by itself once the cause had been treated.
***
Three areas—health policy, education, and drug availability—contribute to the limited availability of palliative care and pain treatment in Ukraine. The World Health Organization sees each of these three areas as fundamental to the development of palliative care and pain management services and has urged countries to take action in each, observing that measures in each area cost little but can significantly impact the availability of palliative care.[22]
Health Policy The WHO has recognized palliative care as an integral and essential part of comprehensive care for cancer, HIV/AIDS, and other health conditions and recommends that countries establish a national palliative care policy or program.[23] While the Ukrainian government has established the Institute of Palliative and Hospice Medicine in the Ministry of Health and created a number of hospices and palliative care beds, no national palliative care policy exists at this time and the government has not undertaken a coordinated effort to address barriers to palliative care. The government’s failure to address critical issues like the lack of oral morphine and the need to develop home-based palliative care are particularly problematic.
Education The World Health Organization recommends that countries adequately instruct healthcare workers on palliative care and pain treatment.[24] Yet in Ukraine official curricula for undergraduate and postgraduate medical studies do not provide any specific education on palliative care and pain management. The WHO cancer pain treatment guideline is barely taught in medical or nursing schools, if it is taught at all. Many healthcare workers interviewed did not understand the basic principles of pain management and palliative care.
Drug availability The WHO recommends that countries establish a rational drug policy that ensures availability and accessibility of essential medicines, including morphine. Under the UN drug conventions countries must ensure adequate availability of opioids for medical purposes while also preventing their misuse.[25] However, Ukraine’s primary focus has been to prevent misuse of these medications. Human Rights Watch recognizes that such prevention is particularly important in countries that, like Ukraine, face major problems with illicit drug use—the country is home to an estimated 230,000 to 360,000 injecting drug users—and corruption in the healthcare sector.[26] But these efforts should not interfere with adequate availability of controlled substances for legitimate, medical purposes.
Ukraine’s drug regulations are far more restrictive than required under the UN drug conventions and contain numerous provisions that directly interfere with the delivery of good pain care, discourage doctors from prescribing opioid medications due to excessively burdensome bureaucratic requirements, and generate fear among doctors of the legal repercussions of prescribing these medications.
To its credit, Ukraine’s government recognizes the need for reform to ensure effective pain treatment and palliative care services. It has established the Institute of Palliative and Hospice Medicines in the Ministry of health, created hundreds of hospice beds, and removed some problematic provisions from its drug regulations in 2010.[27] In an October 2010 meeting with Human Rights Watch the then head of the National Drug Control Committee expressed concern about the lack of narcotics licenses at pharmacies in rural areas and said his committee was exploring solutions.[28]
***
Under the International Covenant on Economic, Social and Cultural Rights, the Ukrainian government is obligated to take steps “to the maximum of its available resources” to progressively realize the right to health. In keeping with this, the government should formulate a plan for the development and implementation of palliative care services, ensure the availability and accessibility of morphine and other medications that the World Health Organization considers essential, and ensure that healthcare providers receive training in palliative care. The Ukrainian government’s failure to do so violates the right to health.
Under the prohibition of torture and ill-treatment, the Ukrainian government has an obligation to take steps to protect people under its jurisdiction from inhuman or degrading treatment, such as unnecessary suffering from extreme pain. As the UN special rapporteur on torture and other cruel, inhuman or degrading treatment or punishment has noted, “failure of governments to take reasonable measures to ensure accessibility of pain treatment … raises questions whether they have adequately discharged this obligation.”[29] The fact that public healthcare facilities in Ukraine offer pain treatment in a way that fundamentally deviates from well-established international best practices and that the government has not taken steps to change this calls into question whether the government has fulfilled this obligation. It may thus be liable under the prohibition of torture and cruel, inhuman, or degrading treatment.
This report focuses specifically on the poor availability of palliative care services in Ukraine. Human Rights Watch fully recognizes the problems that exist with availability and accessibility of other health services in Ukraine. The fact that this report focuses on a specific area of healthcare does not suggest that government authorities in Ukraine do not have an obligation under international human rights law to take reasonable steps to address problems in other parts of the healthcare system.
Key Recommendations
To the Government of Ukraine:
- Ensure the availability of oral morphine throughout the public healthcare system.
- Amend licensing provisions of drug regulations to ensure that all rural healthcare clinics and hospitals can obtain licenses for strong opioid analgesics.
- Amend drug regulations to ensure that patients or their relatives can receive a reasonable take-home supply of strong opioid analgesics that realistically enables them to enjoy continuous pain relief.
- Disseminate WHO pain treatment guidelines to all healthcare facilities and roll out in-service training for all oncologists and other relevant healthcare workers.
In consultation with all relevant stakeholders, develop an action plan to ensure access to palliative care and pain management nationwide that provides for:
- Developing a national palliative care and pain treatment guideline, consistent with international best practices.
- Introducing instruction on internationally recognized pain treatment best practices in all medical and nursing schools and as part of continued medical education programs.
- A review process for Ukraine’s drug regulations aimed at ensuring adequate availability and accessibility of strong opioid medications for medical use while preventing their misuse.
To the Zdorovye Narodu Pharmaceutical Company:
- Amend product information for injectable morphine to bring it in line with available evidence.
- Start manufacturing oral morphine.
To the International Community:
- Raise concern with the government of Ukraine about the limited availability of quality palliative care and pain treatment services.
- Offer technical and financial assistance to implement the recommendations contained in this report.
Methodology
This report is based on research conducted between March 2010 and 2011, including field visits to Ukraine in April and October 2010. Field research was conducted primarily in the Kharkiv and Rivne provinces and in Kiev. Research in these provinces and Kiev was conducted jointly with the Institute of Legal Research and Strategies in Kharkiv and the Rivne and Kiev branches of the All-Ukrainian Network of People Living with HIV. We chose these locations for research because of their geographic diversity. Additional research was conducted in the cities of Lviv and Cherkassy. We also conducted desk research regarding palliative care availability in various other parts of the country.
During four weeks in Ukraine a researcher from Human Rights Watch and each partner organizations conducted more than 67 interviews with a wide variety of stakeholders, including 20 people with cancer, HIV/AIDS, and other life-limiting health conditions, or their relatives; 35 healthcare workers, including oncologists, AIDS doctors, anesthesiologists, palliative care doctors, and administrators of hospitals, hospices, and palliative care programs; and a dozen drug control and health officials.
Most interviews with patients and their relatives were conducted at their homes. Interviews were conducted in private.
Interviews were semi-structured and covered a range of topics related to palliative care and pain treatment. Before each interview we informed interviewees of its purpose, informed them of the kinds of issues that would be covered, and asked whether they wanted to participate. We informed them that they could discontinue the interview at any time or decline to answer any specific questions without consequence. No incentives were offered or provided to persons interviewed.
We have disguised the identities of all patients, relatives, and healthcare workers interviewed to protect their privacy, except when they specifically asked for their identity to be used. Similarly, we have disguised the names of the districts we visited to protect healthcare workers who, as government employees, may have legitimate concerns about a possible negative official response to their speaking out about problems with pain treatment.
All interviews were conducted in Russian by the Human Rights Watch researcher, a fluent Russian speaker. Most interviewees had no difficulty speaking Russian. Researchers from partner organizations provided translation where necessary.
In October 2010 Human Rights Watch presented preliminary findings to the Ministry of Health, the National Drug Control Committee, the section for the licit circulation of narcotic drugs of the Ministry of Interior, and the State Expert Center of the Ministry of Health. In March 2011, Human Rights Watch wrote a detailed letter summarizing the report’s findings to the pharmaceutical company Zdorovye Narodu, inviting it to respond to the findings and to present comments in this report. A copy of the letter is included in this report in Annex 1. No response had been received by the time the report went to print in late April 2011.
All documents cited in the report are publicly available or on file with Human Rights Watch.
I. Overview: Palliative Care and Pain Treatment
Palliative care seeks to improve the quality of life of patients facing life-limiting or terminal illness. Its purpose is not to cure a patient or extend his or her life. Palliative care prevents and relieves pain and other physical and psychosocial problems, “adding life to the days, not days to the life,” in the much-quoted words of Dame Cicely Saunders, founder of the first modern hospice. The World Health Organization recognizes palliative care as an integral part of healthcare that should be available to those who need it.[30] While palliative care is often associated with cancer, a much wider circle of patients with health conditions can benefit from it, including patients in advanced stages of neurological disorders, cardiac, liver, or renal disease or chronic and debilitating injuries.
One key objective of palliative care is to offer patients treatment for their pain. Chronic pain is a common symptom of cancer and HIV/AIDS, as well as other health conditions.[31]Research consistently finds that 60 to 90 percent of patients with advanced cancer experience moderate to severe pain.[32] Prevalence and severity of pain usually increase with disease progression: several researchers have reported that up to 80 percent of patients in advanced stages of cancer experience significant pain.[33] Pain symptoms are a problem for a significant proportion of people living with HIV as well, even as the increasing availability of antiretroviral drugs in middle and low-income countries prolongs lives.[34] With the advent of antiretroviral therapy (ART), the international AIDS community has understandably focused on treatment for people living with HIV. Unfortunately, this has led to a widespread but incorrect perception that these people no longer needed palliative care. In fact, various studies have shown that a considerable percentage of people on ART continue to experience pain and other symptoms that improve with simultaneous delivery of palliative care and ART.[35]
Moderate to severe pain profoundly impacts quality of life. Persistent pain has a series of physical, psychological, and social consequences. It can lead to reduced mobility and consequent loss of strength; compromise the immune system; and interfere with a person’s ability to eat, concentrate, sleep, or interact with others.[36] A WHO study found that people who live with chronic pain are four times more likely to suffer from depression or anxiety.[37] The physical effect of chronic pain and the psychological strain it causes can even influence the course of disease, as the WHO notes in its cancer control guidelines, “Pain can kill.”[38] Social consequences include the inability to work, care for oneself, children, or other family members, participate in social activities, and find emotional and spiritual closure at the end of life.[39]
According to the WHO, “Most, if not all, pain due to cancer could be relieved if we implemented existing medical knowledge and treatments” (original emphasis).[40] The mainstay medication for treating moderate to severe pain is morphine, an inexpensive opioid made of poppy plant extract. Morphine can be injected, taken orally, delivered through an IV, or into the spinal cord. It is mostly injected to treat acute pain, generally in hospital settings. Oral morphine is the drug of choice for chronic cancer pain and can be taken both in institutional settings and at home. Morphine is a controlled medication, meaning that its manufacture, distribution, and dispensing is strictly regulated at both international and national levels.
Medical experts have recognized the importance of opioid pain relievers for decades. The 1961 Single Convention on Narcotic Drugs, the international treaty governing use of narcotic drugs, explicitly states that “the medical use of narcotic drugs continues to be indispensable for the relief of pain and suffering” and that “adequate provision must be made to ensure the availability of narcotic drugs for such purposes.”[41] The WHO includes both morphine and codeine (a weak opioid) in its Model List of Essential Medicines, a roster of the minimum essential medications that should be available to all persons who need them.[42]
Yet, approximately 80 percent of the world’s population has either no, or insufficient, access to treatment for moderate to severe pain and tens of millions of people around the world— including around 5.5 million cancer patients and 1 million end-stage HIV/AIDS patients—suffer from moderate to severe pain each year without treatment.[43]
But palliative care is broader than just relief of physical pain. Other key objectives may include provision of care for other physical symptoms and psychosocial and spiritual care for patients and family members who face life-threatening or incurable and often debilitating illness. Anxiety and depression are common symptoms.[44] Palliative care interventions like psychosocial counseling have been shown to considerably diminish incidence and severity of such symptoms and to improve the quality of life of patients and their families.[45]
Palliative care also seeks to alleviate other physical symptoms, such as nausea and shortness of breath, which are frequently associated with life-limiting illness and significantly impact a patient’s quality of life.
The WHO has urged countries, including those with limited resources, to make palliative care services available. It recommends that countries prioritize implementing palliative care services in the community—providing care in medical institutions that deal with large numbers of patients requiring palliative care services and in people’s homes rather than at healthcare institutions—where it can be provided at low cost and where people with limited access to medical facilities can be reached.[46]
II. Rural Areas: Unavailable or Hard-to-Access Strong Pain Medications
Patients who live in remote places are doomed.
—Nurse, District 1, April 16, 2010.
The Story of Konstantin Zvarich
Konstantin, a 67-year-old pensioner from Poltava province in central Ukraine, was in many ways a typical Ukrainian from the countryside. Born in 1943, he grew up amid the hunger and devastation caused by World War II. As a young man, he served in the Soviet army before joining a collective farm, where he worked for 46 years. Konstantin was married, had a daughter, and a grandson.
Konstantin developed problems urinating in January 2009. When the pain became so severe he could no longer pass urine, he went to the local clinic where a doctor diagnosed prostate cancer. Two rounds of surgery provided temporary relief from the pain but were unable to remove all the cancerous cells which soon began to metastasize.
A sudden onset of intense pain in his hands and fingers alerted Konstantin that all was not right. He had further tests which revealed the cancer had spread to his bones, a condition often associated with severe pain. Konstantin told Human Rights Watch:
The pain was so bad that my whole body seemed to break. We would call the ambulance every two to three hours because I could not stand it.[47]
Doctors briefly hospitalized Konstantin and then sent him home with a prescription for tramadol, a weak opioid pain medication. Because few pharmacies in Ukraine stock tramadol—the result of the government’s 2008 decision to treat tramadol essentially like morphine—Konstantin’s relatives had significant difficulty obtaining the medication. When Konstantin was finally able to get tramadol, it turned out to be far too weak to control his excruciating pain. He used all ten ampoules of tramadol—the maximum allowed under Ukrainian law per prescription—in a day without bringing his pain under control.
Konstantin’s daughter, a medical doctor in Kharkiv, a city in eastern Ukraine, advised him to take a variety of over-the-counter pain medications, none of which provided much relief. Although he complained to his doctors of severe pain, Konstantin’s doctors never prescribed morphine. The local clinic did not have the necessary license. (See Chapter III for detail on licensing requirements and procedures.) For four or five months Konstantin suffered ongoing, severe pain. Describing one particular episode, he broke down in tears saying:
It was unbearable. I came home and the pain grabbed me so strongly. It was so bad that I didn’t know what to do with myself. It is so difficult to live like this.[48]
One day in late summer, Konstantin’s pain was so bad that his grandson, who was staying with him in the village, called his mother, telling her that his grandfather was “bouncing of the walls” from pain. The daughter decided she could no longer leave her father in the village and managed to arrange a bed for him at Kharkiv’s hospice. There, Konstantin finally received morphine for his pain. When Human Rights Watch interviewed him in April 2010, he said that his pain was finally under control at the hospice.
Konstantin died in June 2010 at the hospice.
Lack of Narcotics Licenses at Health Clinics and Pharmacies
Tens of thousands of patients with pain across Ukraine’s vast rural areas, where one-third of its population of approximately 46 million people lives, face a similar fate to Konstantin’s every year.
Local health clinics—known asambulatoria and feldshersko-akusherski punkt (FAP)—and even many small hospitals do not have the narcotics licenses necessary to stock and prescribe strong opioid analgesics.[49] Even when patients receive a prescription, few pharmacies in rural areas are licensed to fill prescriptions for opioid medications. Although most of these facilities could apply for a license, Ukraine’s drug regulations require that they have a separate room to store these medications, which is specially equipped to prevent break-ins and theft. The associated cost and lack of spare rooms are the main reasons that few health clinics and small hospitals obtain narcotics licenses.
During its research, Human Rights Watch and local partners visited the main hospitals in five districts in Kharkiv and Rivne provinces, in eastern and western Ukraine respectively. Known as central district hospitals, these are the main health facilities and administrative centers for the public healthcare system in their districts. While all the central district hospitals had narcotics licenses, doctors at each location told us that none of the clinics did. As Table 1 shows, this means that many patients live dozens of kilometers away from health facilities that are authorized to prescribe strong pain medications, even if their own village or town has a health facility. A health official in Rivne province told us that the same was true for all other districts in the province: all 15 central district hospitals have the license but none of the 92 ambulatorias or the 613 FAPs in the province do.
TABLE 1
District |
Size[50] (in sq. km) |
No. of district hospitals (with license) |
No. of ambulatories (with license) |
No. of FAPs (with license) |
District 1[51] |
467 |
1 (1) |
2 (0) |
5 (0) |
District 2[52] |
1,149 |
4 (1) |
13 (0) |
10 (0) |
District 3[53] |
1,011 |
1 (1) |
6 (0) |
8 (0) |
District 4[54] |
693 |
1 (1) |
4 (0) |
33 (0) |
District 5[55] |
659 |
2(1) |
6(0) |
27(0) |
District 6[56] |
695 |
1 (1) |
43(0) |
6(0) |
A Broken Pain Treatment Delivery System
The lack of licensed health facilities in rural areas means that patients or their relatives have to travel long distances to fill prescriptions for strong pain medications, often on poor roads and infrequent public transport. This already problematic situation is exacerbated by a pain treatment delivery system that does not allow clinics to provide patients and their relatives with a take-home supply of injectable morphine.
In general, doctors do not write prescriptions for strong opioid analgesics for patients to fill at pharmacies. Instead, patients receive morphine from hospital stock. While this has the advantage that patients do not have to pay for their medications, Ukraine’s drug regulations require healthcare workers to administer injectable strong opioid analgesics from hospital stock directly to the patient.[57] In other words, health facilities are not allowed to give the medication to patients or their relatives to take home and administer themselves. Instead, healthcare workers are supposed to visit patients at their homes for every prescribed dose of injectable morphine, often multiple times per day. The regulations do allow self-administration of oral opioid analgesics but none are available in Ukraine due to a lack of effort by the government to offer them through the public healthcare system.
This system is a major barrier to evidence-based pain care in Ukraine’s urban areas and often an insurmountable obstacle in rural areas, where healthcare facilities lack the resources for staff to travel to central district hospitals to pick up strong pain medications and then visit patients at home several times per day. As a result, many patients end up without access to the pain medications they need. Some patients may get pain medications once or twice per day. And only a very few—those who live in districts where doctors are willing to ignore government regulations—might get reasonably effective pain treatment.
In each of the five districts visited for this research, we found that healthcare providers struggled to deliver strong pain medications to patients. Their approaches (Table 2) varied from not providing strong opioid analgesics to patients outside district centers to trying to accommodate them. But even in the best scenario major and unnecessary obstacles remained to evidence-based pain care. In each district, doctors and nurses openly admitted that many patients, particularly those who live outside the district centers, were not getting the care they needed.
TABLE 2
Districts |
Approach to delivering pain treatment |
Districts 1, 2, 5, 6 |
Nurses from the central district hospital are responsible for delivering injections with pain medications during the day to patients who live in the district center; ambulances administer the injections during evening hours. Nurses and feldshers from local health clinics are responsible for delivering pain treatment to patients outside the district center.[58] They have to travel to the central district hospital every day to pick up the daily supply of medications for their patients and then visit them at their homes for each injection. Ambulances do not provide pain care outside the district center. |
District 3 |
Ambulances are responsible for delivering strong pain medications to all patients irrespective of where they live and at any time of the day. |
District 4 |
Healthcare workers provide patients or their relatives with a three-day supply of morphine and allow them to administer the medication themselves, in contravention of Ukraine’s drug regulations. Every third day, in return for the empty ampoules, patients or their relatives receive their medications for the next three days. Nurses and feldshers at local health clinics are instructed to check in on patients regularly to ensure that they are using the medications appropriately and are achieving adequate pain control. |
In districts 1, 2, 5, and 6, patients living outside the district center often had no access to strong opioid analgesics. The oncologist in district 2 expressed his frustration with the system:
Prescribing is not the issue; it’s delivering the medications. If possible, injections are done daily. If the area is close, the nurse can come every day to pick up morphine. They may come in the car of the FAP or ambulatoria and return the empty ampoules the next day. But not all FAP and ambulatoria have cars or they don’t have money for gas … If people live far away, the reality is that we make do with tramadol [a weak opioid] and dimedrol [an antihistamine]. We try the best we can. It is a tragedy for such patients. I look at them and I want to do something for them but I can’t.[59]
He added there were currently 30 end-stage cancer patients in his district, 20 of whom should have been receiving strong opioid analgesics. Instead, only three were.
The oncologist in district 5 said it was problematic for nurses and feldshers to travel to the central district hospital: “The rural healthcare system is poorly funded so they [nurses and feldshers] talk to the relatives who pay for their travel or bring the feldsher in their own cars.”[60] A nurse in district 1 said that staff at health clinics in her district cannot travel to the central district hospital to pick up morphine due to lack of transportation and time.[61] She took us to the house of a cancer patient who had been prescribed one injection of morphine per day, which was delivered via an ambulance that drove to his house every evening. The patient said he was in significant pain during the day and that “it would be very good to have a second injection. They make me feel so much better.”[62] The nurse said this was unlikely to happen:
If they prescribe an injection during the day, I will have to go to the patient’s home myself. That would mean that when I come to work at 8 a.m. I would pick up the ampoule from the chief nurse and then to walk to the patient’s home because there is no transportation. That takes 30 to 40 minutes. I would do the injection and then have to walk back. So it takes more than an hour to do one injection. That’s why we try not to prescribe during the day.[63]
|
Poland’s Rural Areas: A Different Story In Poland, morphine and other strong opioid analgesics are readily available in rural areas. Oral morphine is included in Poland’s essential medicines list, and pharmacies and health clinics are required to stock them. Although pharmacies may apply for a waiver to this rule—and quite a few do for opioid medications—there is a dense network of pharmacies with narcotics licenses throughout the country. When a pharmacy does not have opioids in stock, it can request them from a wholesaler and generally receive new supply within half a day. Opioid medications are provided free of charge at pharmacies. Doctors can prescribe a 30-day supply of oral morphine per prescription. The prescription can be filled at any pharmacy that has a narcotics license. Human Rights Watch interview with Dr. Tomasz Dzierzanowski, palliative care physician |
Most patients in the district, she said, receive just one injection of morphine per day at most, leaving them in pain for most of the day. The nurse noted that occasionally patients get a second injection. She could remember only one patient in her eight years at the hospital who received three injections of morphine per day.[64]
Asked if they ever simply gave ampoules, small glass vials that contain morphine, to patients or their relatives to take home, healthcare workers in districts 1 and 2 said that they never did because of the strict control over these medications. The nurse in district 1 said: “The chief nurse [who is responsible for keeping records] is very strict with narcotic drugs. She has to protect herself because there can be an inspection anytime and if ampoules are missing she is in trouble.”[65] The oncologist in district 5 said that she and her colleagues sometimes provide patients from villages with a two or three-day supply.[66]
In district 3, where ambulances deliver pain medications to all patients, access to pain medications is significantly better. However, the ambulance service is not able to visit patients every four hours, so most receive two doses of morphine per day.[67] The chief doctor noted that poor weather—much of Ukraine sees significant snowfall in winter—can undermine the delivery of pain medications. He recalled difficulties during the winter of 2010 when major snowfall made many roads inaccessible:
If there is no road [accessible] we drive to the farthest point and then go on foot. But in some cases, getting to the village—not to speak of the house—was impossible this winter. We would contact the road service … We had three patients in [rural villages] and asked if they could at least open up the roads there. So we didn’t leave them without help. Of course we couldn’t stick to the timing of the injections.[68]
In district 4, healthcare workers violate Ukraine’s drug regulations—and potentially expose themselves to disciplinary and criminal sanctions—to improve patients’ accessibility to strong opioid analgesics. Here, patients and their relatives are responsible for obtaining pain medications from the central district hospital themselves. This gives them the option of getting more doses of the pain medications, and flexibility to administer it when most convenient for them. But it also places a significant burden on families who have to travel every three days to the central district hospital to collect medications.
The deputy chief doctor at the central district hospital recalled an elderly lady who had been coming to the central district hospital every three days for the last 18 months to pick up pain medications for her husband, a cancer patient. Even though there is a health clinic a kilometer from her house, she has to travel 20 kilometers every third day to the district center because the local clinic does not have a narcotics license.[69] A nurse at the central district hospital recalled a woman who had to leave her village at 4 a.m. every third day in order to catch a minibus to the district center and be able to make it back home the same day.[70]
Svitlana Bulanova told us of her sister’s plight caring for her daughter, Irina, a young woman with cervical cancer. She said that after Irina’s cancer had metastasized to her bones, she often screamed in agony due to the pain. Her doctors prescribed morphine but their local clinic did not have a narcotics license. So Irina’s parents had to travel the 25 kilometers to the district center every third day on public transport, a five-hour round trip that involved walking to the main road, waiting for a mini-bus to the district center, walking to the central district hospital to get the medications, and then repeating the journey on the way back.[71]
Pharmacies and Opioid Analgesics
Pharmacies play a limited role in distributing strong opioid analgesics to patients in Ukraine because most doctors prescribe these medications from hospital stock. But they do play a significant role in distributing tramadol, a weak opioid analgesic widely used in Ukraine.
Pharmacies must have a narcotics license before they can stock and dispense medications like morphine or tramadol. Yet, few pharmacies in rural areas have such licenses. The head of Ukraine’s National Drug Control Committee, the government agency responsible for issuing licenses, told Human Rights Watch in October 2010 that in Kirovohradskaia province there were only four pharmacies with such a license for 1.1 million people.[72] He said the situation in other provinces was somewhat less extreme but still highly problematic.[73]
This means that patients or their relatives often have to travel to district centers to fill their prescriptions, encountering the same challenges as described above. The chief doctor at the central district hospital in district 3, for example, said that in his district there is not a single pharmacy with a narcotics license so patients have to travel to the next district to fill prescriptions for tramadol. The doctor noted that there are just two buses per day to the town, making the trip very burdensome for people without their own transportation.
Ukraine’s drug regulations impose a strict limit on the amount of medication that can be prescribed per prescription. Table 3 shows the maximum amounts for several medications commonly used in pain management. This means that the patient or relatives have to obtain a new prescription every few days and then travel to the licensed pharmacy to fill it.
TABLE 3
Medication |
Maximum amount per prescription[74] |
In special cases of “lingering and chronic forms of disease” |
Typical daily dose |
Average time covered per prescription (in special cases) |
Tramadol injectable (1 ml – 50 mg; 2 ml – 100 mg) |
10 ampoules |
20 ampoules |
Up to 600 mg/day |
2 (4) days |
Tramadol tablets (50mg) |
30 tablets |
60 tablets |
Up to 400 mg/day |
4 (8) days |
Morphine (8.6 mg) |
10 ampoules |
20 ampoules |
20-25 mg / day[75] |
4 (8) days |
III. Throughout Ukraine: Ensuring Quality of Pain Treatment Services
The Story of Lyubov Klochkova
Lyubov, a woman in her mid-forties, was a tireless advocate for health rights. In her native city in Western Ukraine, she set up and ran successful health and legal service programs. But she spent much of her time traveling around Ukraine, Russia, and other parts of the former Soviet Union to share her expertise with others.
In 2008, as she was attending a conference, Lyubov suddenly felt desperately ill. Back home, medical tests found metastatic cervical cancer for which she was immediately treated. Several months later Lyubov returned to work; doctors thought her cancer was in remission.
But in early 2009 it became clear that all was not well. Rarely sick before, Lyubov now suffered colds that she could not seem to shake. By March a problem urinating sent her back to her doctor. Examinations showed that her cancer had recurred and that a tumor was blocking her kidney.
At around the same time Lyubov developed increasingly severe pain. At first her doctors tried to treat it with over-the-counter drugs and weak opioids that provided limited relief. Although her doctor recommended morphine Lyubov was ambivalent. She was worried that her body would get used to the medication and it would not be effective when she needed it most. A stoic woman, she continued to work, taking taxis to meetings to avoid having to walk. But by the end of May she had become too sick to leave the house.
With the pain now too great to bear, Lyubov agreed to take morphine.[76] “Why did I doubt for so long whether or not to start morphine?” she said when she got her first dose.
But the relief did not last long. Her doctor had prescribed one shot of morphine per day giving her relief for just about four hours. Over the next few weeks, as Lyubov kept complaining of persistent pain, doctors added an extra shot each week until she finally received five ampoules of morphine per day. Every morning, a nurse would visit the apartment and, in violation of Ukraine’s drug regulations, left the supply of morphine for the day. Lyubov’s husband would administer the medication when she needed it.
But five ampoules per day were not sufficient to control Lyubov’s pain. Her relatives were forced to ration the medication for when she needed it most. Lyubov would try to tolerate her pain. Her daughter told Human Rights Watch:
The daily dose was sufficient at most for three [effective] doses; in other words, for twelve hours. Because they brought us too little morphine we tried to save most of it for the night. During the day, we gave her drugs from the pharmacy and a minimal dose of morphine. Most of it we left for the night.[77]
By the morning, the morphine would be finished and Lyubov would anxiously wait for the nurse to come. Lyubov’s daughter said:
The nurse [normally] came at 10 or 11 a.m., but sometimes she was late. Mama would slumber at night. By 8 a.m. she would sit up rigid [from the pain] and wait for the nurse to [arrive with the morphine].[78]
A few weeks before her death Lyubov made an unpleasant discovery: she had reached the maximum daily dose for morphine and her doctor would not be able to prescribe any more ampoules. As Lyubov’s pain intensified the five ampoules gave her less and less relief. Lyubov and her daughter left no stone unturned trying to get a larger morphine dose:
We of course asked for a sixth ampoule. When they told us that five was the maximum we tried to find out through [a palliative care expert] whether that’s true, how that’s determined, and how we could get more of the medicine. Unfortunately, nothing worked out. The doctors said that they don’t have the right to prescribe more. We discussed it with the oncologist, the gynecologist, with all of them. We tried to mobilize everyone we could.[79]
But the doctors would not budge. Lyubov had to somehow make do with an increasingly inadequate amount of morphine. For several weeks she faced great suffering until, during her last few days, her kidneys could no longer clear the morphine from her body and her pain seemed to subside. She died in late July 2009.
Comparing Ukrainian Pain Treatment Practices with WHO Principles
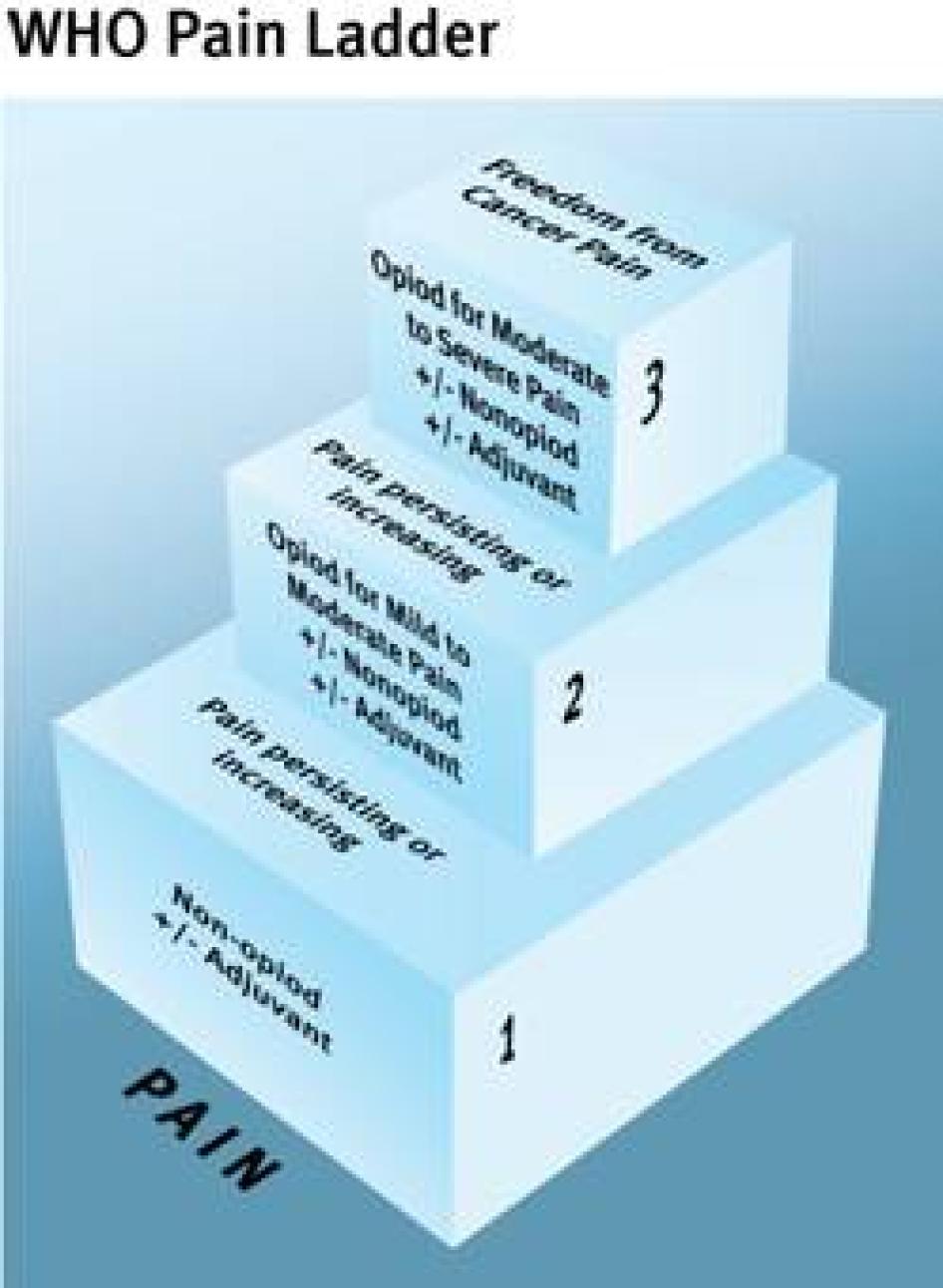
The WHO Cancer Pain Ladder, a treatment guideline first published in 1986, is an authoritative summary of international best pain treatment practices available.[80] Based on a wealth of pain treatment research that spans decades, it has formed the basis for cancer pain treatment in many countries around the world. It has also been used successfully to treat other types of pain.[81] The treatment guideline is organized around five core principles for treating pain (see Table 4). The European Society for Medical Oncology (ESMO) and the European Association of Palliative Care (EAPC) have also developed cancer pain treatment guidelines, which follow these same core principles.[82] If followed, WHO estimates, the ladder can result in good pain control for 70 to 90 percent of cancer patients.[83]
Our research has found that standard pain treatment practices in Ukraine deviate fundamentally from World Health Organization recommendations, with all five core principles articulated in the treatment guideline widely ignored.
Under the right to health, governments must ensure that pain treatment be not only available and accessible, but also that it be provided in a way that is scientifically and medically appropriate and of good quality.[84] This means that healthcare providers should provide pain management in a way that is consistent with internationally recognized best practices. Governments, in their turn, have to create conditions which allow healthcare providers to do so.
TABLE 4: Comparing the Core Principles of Cancer Pain Treatment with Ukrainian Pain Treatment Practices
WHO Recommendation |
Ukraine’s Practice |
Principle 1: Pain medications should be delivered in oral form (tablets or syrup) when possible. |
Patients receive morphine by injection only. |
Principle 2: Pain medications should be given every four hours. |
Most patients receive morphine once or twice per day, in exceptional cases three or four. |
Principle 3: Morphine should be started when weaker pain medications prove insufficient to control pain. |
Patients are often started on morphine only when curative treatment is stopped, irrespective of pain levels. |
Principle 4: Morphine dose should be determined individually. There is no maximum daily dose. |
Patients are routinely injected with one ampoule of morphine at the time, irrespective of whether this is too little or too much. Many Ukrainian doctors observe a maximum daily dose of 50 mg of injectable morphine, even if it is insufficient to control the patient’s pain. |
Principle 5: Patients should receive morphine at times convenient to them. |
Administration of morphine depends on work schedules of nurses. |
Principle 1: “By Mouth”
If possible, analgesics should be given by mouth. Rectal suppositories are useful in patients with dysphagia [difficulty swallowing], uncontrolled vomiting or gastrointestinal obstruction. Continuous subcutaneous infusion offers an alternative route in these situations. A number of mechanical and battery operated pumps are available.
—WHO Treatment Guideline[85]
The first principle of the WHO cancer pain treatment guideline reflects a fundamental principle of good medical practice: the least invasive medical intervention that is effective should be used when treating patients. As injectable analgesics provide no benefit over oral pain medications for most patients with chronic cancer pain, the WHO recommends the use of oral medications. Also, using oral medications eliminates the risk of infection that is inherent in injections and is particularly elevated in patients who are immuno-compromised due, for example, to HIV/AIDS, chemotherapy, or certain hematologic malignancies. When patients cannot take oral medications and injectable pain relievers are used, it recommends subcutaneous administration (under the skin) to avoid unnecessary repeated sticking of patients.[86] Hence, oral morphine, which the WHO considers an essential medicine that must be available to all who need it, is the cornerstone of the treatment guideline.[87]
In Ukraine, however, oral morphine is not available at all. In fact, it is not even a registered medication. A recent survey of European countries found that Armenia, Azerbaijan, and Ukraine are the only countries in Europe where oral morphine is altogether unavailable. Armenia is currently looking for a supplier of oral morphine.[88] The only non-injectable strong opiod analgesics available in Ukraine are Fentanyl patches that release the analgesic through the skin but at a cost of about 267 to 467 hryvna (US$33.75 to 58.38) per patch (active for three days). They are unaffordable for most Ukrainians and are not available in government clinics and most pharmacies.[89]
While the WHO recommends that injectable pain relievers should be injected under the skin, standard practice in Ukraine is to give morphine by intramuscular injection. This means that patients who get morphine every four hours, as recommended, are unnecessarily injected six times per day. On average, patients with advanced cancer who have severe pain require 90 days of treatment with morphine, so a typical patient receiving morphine every four hours would get injected in the muscles 540 times over that period. In interviews, patients and their families said that receiving multiple injections in the muscles was unpleasant, but they were also resigned to the fact that the alternative—unrelieved cancer pain—was far worse.
Patients who are emaciated due to their illness face particular difficulties with intramuscular injections as they have little muscle tissue left. In such patients it may be challenging to vary the place of injection and there is a risk that part of the morphine will end up outside the muscle tissue, resulting in poor absorption of the medication and inadequate pain control. In interviews, both healthcare workers and patients spoke of these difficulties. Lyubov’s daughter, for example, told Human Rights Watch:
The last two weeks we didn’t inject in the behind anymore. The morphine was no longer absorbed. So we started doing intravenous injections in the hand but that’s painful … Of course, if you compare the pain from the injection to the cancer pain it’s not comparable…[90]
Vlad’s mother, Nadya, said that multiple injections of morphine and other medications over the course of several years had turned her son’s behind into a “mine field.” “There was nowhere to inject anymore. It no longer absorbed the medication. The last months we injected in the legs, from the thigh to the knee and in the hand,” she said. One of the injection sites became infected and developed a small hole in the hand. “We only just cured it when he died.”[91] Svitlana Bulanova said that toward the end of her niece Irina’s life, they “had no place left to inject.”[92]
Healthcare workers acknowledged occasional problems due to emaciation. Some said that they alternated the place of injection in such cases. For example, a nurse in district 4 said that they would do one injection “in the shoulder, another in the hip. We switch around.”[93] Several others said that they would switch to subcutaneous injections in such situations.[94] Most healthcare workers we interviewed said that they wished they had oral morphine tablets, saying it would significantly simplify their work. The oncologist in district 5 said: “Patients often ask for strong pain medications in tablets but we [don’t have them].”[95]
Principle 2: “By the Clock”
Analgesics should be given “by the clock,” i.e. at fixed [four hour] intervals of time. The dose should be titrated against the patient’s pain, i.e. gradually increased until the patient is comfortable. The next dose should be given before the effect of the previous one has fully worn off. In this way it is possible to relieve pain continuously.
Some patients need to take “rescue” doses for incident (intermittent) and breakthrough pain. Such doses, which should be 50-100% of the regular four-hourly dose, are in addition to the regular schedule.
—WHO Treatment Guideline[96]
The second principle reflects the fact that the analgesic effect of morphine lasts four to six hours. Thus patients need to receive doses of morphine at four-hour intervals to ensure continuous pain control.
This principle is not followed in rural areas because of the requirement in Ukraine’s drug regulations that a healthcare provider administers the morphine to the patient.[97] Our research also found the same to be true in urban areas. Even in places where population density is much greater and distances smaller, Ukraine’s healthcare system does not have the capacity—or is unwilling to dedicate the resources—to visit patients at home every four hours. So most patients get just one or two doses of morphine, leaving them without adequate pain control for sixteen to twenty hours every day. Even the “lucky” patients who get three or four doses of strong pain relievers daily face significant intervals between injections when their pain is not properly controlled.[98]
Table 5 shows the frequency with which morphine injections are provided to out-patients through a number of hospitals that we and our partners visited.
TABLE 5
Hospital |
Maximum frequency |
Delivery System |
Therapeutic department of a Kharkiv polyclinic |
Nor more than two injections per day. |
A team of nurses and drivers delivers pain medications to patients. |
Rivne polyclinic |
Generally two injections, morning and evening. Maximum is four. |
A team of nurses and drivers delivers the injections to patients. |
District 1 |
Generally one injection, rarely two. |
Ambulance delivers injection in evening. If second injection is prescribed, nurse has to administer. |
District 2 |
One or two. |
Ambulance delivers injection in evening. If second injection is prescribed, nurse has to administer. |
District 3 |
Up to three. |
Ambulance delivers throughout district. |
District 4 |
Three to five. |
Ampoules are given to patients or relatives for self-administration. |
District 5 |
One or two (up to six if nurse offers take-home supply). |
Nurses visit; occasionally, a take-home supply is provided. |
District 6 |
One or two. |
A team of nurses and drivers delivers injection to patients at home but only in the district town. |
While the requirement that healthcare workers administer every dose of morphine to the patient poses the greatest barrier to following the WHO recommendation that morphine be administered every four hours, insufficient training of healthcare providers is another significant obstacle.
Our interviews with healthcare workers suggest that most are unaware of the WHO’s recommendation for four-hourly administration of morphine. Standard procedure appeared to be to start patients on a single shot of morphine in the evening and then add a second injection and more if patients complain of persistent pain. None of the healthcare workers interviewed felt that this was inappropriate or substandard medical practice. For example, the nurse at a polyclinic in Rivne told us:
Patients generally get two ampoules per day: in the morning and evening. It usually begins with an evening dose at 9 or 10 p.m. Sometimes it happens that the next day, the patient already asks for more because it was enough for the night but [not for] the whole day … Before 10 p.m. severe pain syndrome begins again. Then a new prescription is prepared for an extra dose.[99]
A man whose mother died of cancer in 2008, explained how doctors prescribed morphine:
They registered us. Then the panel of doctors met [to discuss my mother’s case] and a decision was made to prescribe morphine. At first… one injection per day. Then, if after a week it isn’t enough in the opinion of the panel, the dose is increased. So there is a correction of the dose over time. So we eventually got two milliliters per day, one milliliter in the morning, one in the evening.[100]
Bridging the Intervals between Morphine Injections
|
The Case of Tamara Dotsenko: The Difference Regular Administration Can Make Tamara Dotsenko, a 61--year-old breast cancer patient, developed severe pain in her spine and back when her cancer metastasized to the spinal cord. In her home village, the health clinic managed her pain by giving her an injection in the evening. Tamara told Human Rights Watch: “In the evening they would give me a shot. I would sleep well and didn’t feel pain. But then during the day it was a different story: pain, pain, pain and pain … I wanted to cry the whole time …” The pain medications they gave her during the day wore off too quickly to provide much relief. When Tamara could no longer take care of herself, she was referred to the hospice in Kharkiv. There, she got pain medications regularly. She said: “Here I get totally different pain treatment. Every six hours they give me an injection. It does not fully control my pain but it is much better than what I had at home. It’s better than having to bear that pain.” Human Rights Watch interview with Tamara Dotsenko (not her real name), Kharkiv, April 16, 2010. |
Healthcare workers and patients told Human Rights Watch that they use a large array of medications, including basic pain medications, weak opioids, muscle relaxants and sedatives, to try to dull the pain in the intervals between morphine doses. For example, a nurse at a polyclinic in Kharkiv told Human Rights Watch: “We never visit patients more than twice a day [to administer morphine]. But a regular nurse will visit to do other injections, other analgesics or muscle relaxants.”[101] She added, erroneously: “After all, morphine … injecting it three times per day is not really all that recommended.” The oncologist in district 3 said that if the three injections of morphine that the ambulance service can deliver each day are insufficient, “we use cocktails: dimedrol with analgin [an antihistamine with a weak pain medication], baralkhin [a weak pain medication], sibazon [diazepam, a sedative].”[102]
While the WHO treatment guideline provides for the use of weak pain medications and other adjuvant medications in addition to a strong opioid analgesic to enhance its analgesic effect or treat specific problems, they are not recommended to be used as an alternative as they are incapable of providing adequate relief.[103] Medications like antihistamines and tranquillizers may be appropriate to treat specific health conditions, such as allergies, nausea, or anxiety, but in Ukraine they appear to be used often primarily to make patients drowsy and dull the pain. Such use is not consistent with the WHO treatment guideline.
Principle 3: “By the Ladder”
The first step is a non-opioid. If this does not relieve the pain, an opioid for mild to moderate pain should be added. When an opioid for mild to moderate pain in combination with non-opioids fails to relieve the pain, an opioid for moderate to severe pain should be substituted. Only one drug of each of the groups should be used at the same time. Adjuvant drugs should be given for specific indications…
If a drug ceases to be effective, do not switch to an alternative drug of the same efficacy but prescribe a drug that is definitely stronger.
—WHO Treatment Guideline[104]
According to the WHO guideline, the intensity of the pain should determine what type of pain medications a patient receives.
For mild pain, patients should receive over-the-counter medications like Ibuprofen or Paracetamol; for mild to moderate pain weak opioid, like codeine; and for moderate to severe pain a strong opioid, like morphine. If over-the-counter pain medications or weak opioids are ineffective, a stronger type of pain medications should be provided. In the words of the guideline, “the use of morphine should be dictated by the intensity of pain, not by life expectancy.”[105]
Leading pain experts have estimated that about 80 percent of terminal cancer patients will require morphine for an average period of ninety days before death.[106] But data we collected from several districts in Ukraine, including districts where hospitals have narcotics licenses, suggest that far fewer than 80 percent of terminal cancer patients get morphine and that those that do generally receive it for far less than 90 days. This suggests that many patients in Ukraine who face moderate to severe pain are started late on morphine or do not receive the medication at all even when it is available. The data is shown in Table 6.
Interviews with healthcare workers support this conclusion. For example, a doctor at a specialized cancer hospital said:
We try to use morphine very rarely because, as all narcotics, it suppresses the breathing center. For cancer patients that is not desirable so it is a last resort. No more than 15-20% of [terminal cancer] patients get it. Generally, we try to make do with non-opioid analgesics or with synthetics…[107]
The doctor’s reluctance to use morphine is based on a misconception about the medication’s effects on the breathing center. According to the WHO:
pain is the physiological antagonist to the central depressant effects of opioids. Clinically important respiratory depression is rare in cancer patients because the dose of the opioid is balanced by the underlying pain.[108]
TABLE 6
District |
Population[109] |
Cancer Registry |
Cancer Mortality for 2009 |
Actual Number of Patients Who Received Morphine |
Percentage of Terminal Cancer Patients who Received Morphine |
Average Time Period Morphine Received |
Therapeutic department of a polyclinic in Kharkiv |
22,000 |
109 |
23 |
4 |
17 |
2-3 months |
District 1 |
11,824 |
252 |
19 |
5 |
26.3 |
No data |
District 2 |
50,945 |
1,608 |
100 |
30 |
30 |
No data |
District 3 |
36,531 |
670 |
100 |
33 |
33.3 |
1.5-2 months |
District 4 |
31,235 |
679 |
66 |
11 |
157 days |
|
District 5 |
58,805 |
1,000 |
103 |
3 |
101 days |
|
District 6 |
117,863 |
1,608 |
170 |
19 |
11 |
40 days |
Pain does not just affect terminal cancer patients: a 2007 review of pain studies in cancer patients found that more than 50 percent of all cancer patients experience pain symptoms.[112] Testimony from healthcare workers suggests that doctors rarely prescribe morphine to patients who are still receiving curative treatment. A doctor at a polyclinic in Kharkiv, for example, told Human Rights Watch that “the prescribing of a narcotic drug is usually reserved for terminal patients.”[113] The doctor from Rivne said that “pain [in patients still receiving curative treatment] is mostly treated with curative interventions, with chemotherapy or radiation.”
A doctor at an inpatient medical oncology unit at the same hospital told Human Rights Watch that she believes that patients who need strong opioids “are not in my patient profile. We are not a hospice. Symptomatic treatment happens at home [after release from the hospital].”[114] Although she acknowledged that she frequently encounters severe pain in her patients, particularly those with bone metastases, she rarely prescribes morphine. She told Human Rights Watch: “We give non-opoid medications like kitonol or dexalgin [weak pain medications]. If people have a clear pain syndrome, we give tramadol. We try to avoid narcotics.” The doctor estimated that only one patient in the past six months had been prescribed morphine or an opioid of similar strength.
The reluctance of doctors to prescribe strong opioids to patients who are still receiving curative treatment appears to be related to fears that patients will become drug dependent. However, these fears are unfounded, and the WHO treatment guideline states that “wide clinical experience has shown that psychological dependence [drug dependence] does not occur in cancer patients as a result of receiving opioids for relief of pain.”[115]
Development of physical dependence and tolerance to morphine does occur but, according to the treatment guideline, are “normal pharmacological responses” and “do not prevent the effective use of these drugs.” If curative treatment successfully addresses the source of the pain, the use of opioids can be tapered and, eventually, stopped.[116]
Principle 4: “For the Individual”
There are no standard doses for opioid drugs. The “right” dose is the dose that relieves the patient’s pain. The range for oral morphine, for example, is from as little as 5 mg to more than 1000 mg every four hours. Drugs used for mild to moderate pain have a dose limit in practice because of formulation (e.g. combined with ASA or paracetamol, which are toxic at high doses) or because a disproportionate increase in adverse effects at higher doses (e.g. codeine).
—WHO Treatment Guidelines[117]
Pain is an individual experience. Different people perceive pain differently; they metabolize pain medications in different ways; and cancers vary from person to person, leading to vastly divergent types and intensities of pain. With so many variables, only an individualized approach to pain treatment can ensure the best relief to all. The WHO therefore recommends that doctors “select the most appropriate drug and administer it in the dose that best suits the individual.”[118]
However, our research suggests that this recommendation is routinely ignored in Ukraine. Many doctors start patients on a standardized dose of morphine—one that, paradoxically, is unnecessarily high for many—and some arbitrarily cap the daily dose of injectable morphine at a maximum of 50 mg, as wrongly recommended by the Ministry of Health and the manufacturer, even if that is inadequate to control the patient’s pain. Both constitute poor medical practice that leads to unnecessary patient suffering.
Standardized Starting Dose
Finding the right dose of morphine for the individual patient is crucially important: if the dose is too low, the patient's pain will be poorly controlled, if too high, the patient will experience unnecessarily severe side effects, including drowsiness, constipation, and nausea. With the right dose, relief is maximized, side effects are minimized, and any drowsiness or confusion should clear up within three to five days.
However, our research suggests that it is common practice in Ukraine for doctors not to determine the appropriate dose on an individual basis. Instead, they prescribe one ampoule of morphine, which contains 8.6 mg of injectable morphine, equivalent to 25.6 mg of oral morphine.[119] This means that some patients receive too much morphine and face needlessly debilitating side effects, while others receive doses that are too small to give full relief.
Viktor Bezrodny, a man whose mother died of gallbladder cancer, told Human Rights Watch that doctors never tried to establish the right dose of morphine for his mother but just prescribed the standard dose of one ampoule. But the morphine injections made her drowsy. He said:
She would sometimes refuse the injection because she didn’t want that state of cloudiness. She kept it [morphine injection] as a last resort. She would say: ‘Let’s take these drops … everything hurts but let’s do the injection later.’[120]
Bezrodny, himself a doctor, told us he doubted any doctor would prescribe part of an ampoule: “If I prescribe a half ampoule I have to somehow account for the rest…”[121]
Roman Baranovskiy, whose mother-in-law died of metastatic lung cancer in 2009, told us that he divided the ampoules himself and injected them in installments. His mother-in-law’s hospital allowed patients to take home a three-day supply of morphine and administer it themselves. He said:
I did not inject two ampoules right away [as prescribed]. I divided them. If you give a large dose, the person falls asleep … [People with pain] when they get relief will relax anyway and become sleepy. But when the person fades and can’t open their eyes, that’s unnecessary. Even one ampoule was sometimes too much.[122]
Most doctors interviewed said they never prescribe partial ampoules but contended that the practice of first prescribing omnopon or promedol, opioid analgesics that are less potent than morphine, constituted a form of titration. A cancer doctor in Rivne, for example, said that he does not prescribe half ampoules because of the need to account for the other half. But he said that he usually starts by prescribing omnopon and promedol and only prescribes morphine when these are no longer effective.[123]
Maximum Daily Dose
While the WHO treatment guideline specifies that the ‘right’ dose is one that “relieves the patient’s pain” and that some patients may need “more than 1000 mg [of oral morphine] every four hours,” the Ukrainian manufacturer of morphine and Ukraine’s Ministry of Health both recommend a maximum daily dose of 50 mg of injectable morphine, equivalent to 150 mg of oral morphine.[124]
The maximum daily dose recommendation is particularly problematic because it is very low. Since most patients require 10-30 mg of oral morphine every four hours, or 60 to 180 mg per day, even patients who fall on the high end of this typical range in Ukraine exceed the maximum dose recommendation if they get their medications every four hours.[125] Doctors at hospices in Kharkiv and Ivano-Frankiivsk, which observe WHO’s recommendations, estimated that about 10 percent of their patients require more than the maximum dose recommended.[126] A 2010 Human Rights Watch survey of barriers to palliative care found that Ukraine and Turkey were the only two of ten European countries surveyed to impose a maximum daily dose for morphine.[127]
Asked whether they followed the recommendation, doctors’ responses varied greatly. Some said that they did not, while other insisted that they had to. One doctor, for example, told Human Rights Watch that his polyclinic ignores the maximum dose recommendation, citing what he called a “basic principle in medicine” that “no matter what the health condition is, patients should not suffer.”[128] Another oncologist said: “It’s possible [to prescribe more] when patients need it. The main thing is to professionally justify the prescription in the patient’s file so as to avoid problems with inspections.”[129] He recalled a patient who had been on 12 ampoules (103.2 mg) of morphine daily for a five-year period.[130] But the oncologist in district 3 said that his clinic cannot prescribe more than what is recommended, even though some of his patients, primarily those with metastases in the bones, cannot achieve good pain control within the recommended daily dose. He said:
Often these patients are in the hospital. There, they receive narcotics three or four times [per day] and [healthcare workers] constantly provide additional analgesics: weak, strong analgesics. They mix. But we never prescribe more than recommended.[131]
The oncologist also expressed the erroneous opinion that giving more than the recommended daily dose would be ineffective and negatively impact the patient’s breathing and organs.[132] As Lyubov’s case demonstrates, where doctors do strictly follow the recommendation, the result can be great suffering.
But our research found that some doctors are even reluctant to prescribe 50 mg of injectable morphine daily. Vlad’s mother had great difficulty getting doctors to prescribe her son more than three ampoules of morphine, even though he continued to have excruciating pain. She described the battles she had to fight:
I demanded a fourth ampoule because he was in bad shape. A panel of doctors came to our house. The chief doctor … took off his underpants, lifted up his clothes, and checked whether he was abusing drugs. Then she accused me of selling drugs.[133]
Rather than recognize the morphine was insufficient for controlling his pain, doctors first accused Vlad of being a drug addict, then his mother of selling drugs. She told Human Rights Watch that she finally went to the city health department and a member of the local parliament to receive permission to switch to a different hospital.
Eventually, doctors prescribed Vlad a fourth ampoule, but even that was insufficient to control his pain and his mother had to again fight doctors to prescribe a fifth:
I went to the chief doctor [of the hospital], the chief medical officer. [There was] again a scandal. The doctors said: ‘A fifth ampoule is an overdose [is too much]. Michael Jackson died of an overdose. Now they’re prosecuting an innocent doctor. And no one is supporting that doctor. It’ll be like with Michael Jackson.’ And I said: ‘But he screams from the pain, disturbs the neighbors; you don’t know how he howls, how much pain he has. People [neighbors] hear how he howls in the apartment. I can’t be in the apartment. I will go crazy the way he howls.’[134]
Finally, the hospital sent a group of doctors to their apartment to determine whether a fifth ampoule was really needed. Vlad’s mother said:
After the visit, there was silence…. I waited and waited and they did not bring the fifth ampoule. I went to the neurologist and said: ‘You’ve seen him. Can’t you talk to the chief doctor?’ He did and they finally gave us the fifth ampoule.[135]
Principle 5: “Attention to Detail”
Emphasize the need for regular administration of pain relief drugs. Oral morphine should be administered every four hours. The first and last dose should be linked to the patient’s waking time and bedtime. The best additional times during the day are generally 10:00, 14:00 and 18:00. With this schedule, there is a balance between duration of analgesic effect and severity of side effects.
—WHO Treatment Guideline[136]
To ensure quality of life for patients with pain, it is not just important to get pain medications regularly but to get them at times that fit their schedule. In order to maximize sleep at night, for example, patients should take their medications shortly before bed time.
However, several healthcare workers and patients told us that the last injection of morphine would typically be scheduled for 6 to 8 p.m. to accommodate nurses’ shifts. As morphine acts for just four hours, that means that the effects will have worn off for these patients before midnight, setting them—and their relatives—up for a restless night. When Vlad was receiving three ampoules of morphine per day, for example, healthcare workers determined that he would receive his injections at 9 a.m., 2 p.m., and 6 p.m. His mother said:
He often didn’t sleep at night. He’d be in agony because of the pain. Then he would sleep long in the morning. So they would arrive at 9 a.m. and he would be asleep. I would say: ‘Leave the medication. I’ll take the syringe. When he wakes up, that’s when it’s important for us to give him the injection. He’s still sleeping.’ [But they would wake him up and] he would say: ‘Nothing hurts right now. I’m sleeping. I don’t need it.’ But they, like zombies, would insist: ‘No, it’s necessary. We will not come another time. Your prescribed time is 9 a.m. So they would inject him while he was sleeping because they had to do the injection and leave.
The chief doctor in district 3 acknowledged the importance of providing pain medications when the patients need it most. He said that his hospital tried to accommodate patients as much as possible:
At the request of relatives, we can do injections until 10 p.m. but not later … In the terminal stages the medication is not sufficient if you give injections at 6 a.m., noon and 6 p.m. By midnight, he will be screaming.[137]
But he noted that in places where regular nurses and drivers employed by clinics, as opposed to the ambulance service, are responsible for delivering pain medications, it becomes difficult to deliver them that late:
The driver works a specific shift. [What happens] if morphine is prescribed for 6 p.m. and the driver’s shift is over at 2:30 p.m. Why does he have to work after hours? Or someone needs to pay him extra. But with our budget deficits…[138]
Some doctors and nurses told us they tried to accommodate their patients by leaving ampoules or filled syringes with them or their relatives, even though this violates Ukraine’s drug regulations. In such cases, patients can choose themselves the best time to take the medication. For example, Viktor Bezrodny told Human Rights Watch:
The nurse would come. In principle, she was supposed to do the injection but she came at a time that was good for her but when, for example, my mother might sleep. [She allowed me] to load the morphine into the syringe and give her the injection when she actually needed it.[139]
Problems with Treatment of Non-Cancer Pain
While pain treatment for cancer patients in Ukraine is severely inadequate, it is even worse for other types of patients due to a lack of recognition amongst healthcare workers that severe pain is common in people who suffer other health conditions and should be treated.
Our research found that doctors are often unwilling to treat such pain, preferring to treat its cause. Under international human rights law, all patients facing severe pain have an equal right to pain treatment, irrespective of the type of underlying illness or condition.[140] The story of Oleg illustrates the problems that many of these patients face.
The Story of Oleg Malinovsky
Oleg Malinovsky with his dog before he became ill. Courtesy of Malinovsky family.
Oleg, a 35-year-old man from Kiev, has been diagnosed with chronic hepatitis C and a range of other illnesses. Oleg’s acute medical problems started in early 2008, shortly after he began treatment for a hepatitis C infection. When he developed numbness in several fingers, doctors hospitalized him for tests and treatment. At the hospital, he contracted a staphylococcus infection, developed recurring high fevers and experienced increasingly severe pain in his hip joints. The treatment he received was not effective. On the contrary, his problems rapidly worsened.
A degenerative process had started in his joints. Oleg’s pain then spread to his lower spinal area before rapidly worsening in July 2008, several weeks after doctors started rheumatology treatment. As any movement of his hips and knee joints caused severe pain, Oleg was forced to lie completely still in his bed throughout the day. His wife told us:
The pain was intolerable with any movement and became more severe with every day because of the pathological process in his hip joints. The pain affected his sleep, appetite, and his psychological condition. He became very irritable and nothing could make him happy anymore. A normal sneeze or cough caused him terrible pain … You could knock on the wall, and if he was lying over there, he would scream [in pain]...[141]
At the Kiev City Rheumatology Center, where he was being treated, doctors eventually agreed to give Oleg a small daily dose of morphine to allow him to sleep at night. But he still faced undiminished pain at other times of the day.
In March 2009 doctors surgically removed portions of the bone in his stiffened joints, resulting in a reduction of pain and some restored mobility. But in September 2009 Oleg again developed persistent and severe pain, this time involving his wrists and elbows. Again, he had to keep completely still in bed. He was unable to move his limbs, preventing him from any activity whatsoever, including eating, washing, or reading. Oleg routinely screamed in pain. Sometimes, the neighbors would knock on the walls because he disturbed them. Oleg repeatedly told his wife that he wanted to die because he could no longer bear the pain.
Over the next seven months Oleg and his wife repeatedly told doctors at their public hospital about the pain he was suffering and asked them to prescribe appropriate pain medications. But instead of prescribing morphine, which had been effective before, his doctors procrastinated. They sent Oleg to a psychiatrist to assess whether his depression and irritability were related to an underlying psychiatric condition, and they sent him to drug treatment doctors because they thought he was addicted to morphine, even though he had not had any in more than six months.
When the psychiatrist and drug treatment doctor confirmed that Oleg suffered from symptom-related depression rather than a mental disorder and ruled out drug dependence, the chief of the clinic promised to prescribe stronger pain medications. Nothing happened.
Eventually, in March 2010, Oleg’s pain improved somewhat on its own. He never got strong pain medications, continues to be bedridden, and experiences significant pain when he moves. Oleg and his wife have filed complaints with the prosecutor’s office and courts in Ukraine about the denial of appropriate pain treatment. So far, the courts have refused to consider the complaints and the prosecutor’s office has not opened an investigation.
|
Treating Pain in Patients with a History of Illicit Drug Use Patients with severe pain who use illicit drugs or have in the past pose a challenge to healthcare providers. These patients have a right to pain management, including with strong opioid analgesics where clinically appropriate, just as any other patient does. But physicians need to pay special attention to ensure that the pain treatment these patients receive is effective and to minimize the risk of misuse of medications. At present, there are no international guidelines for treating pain in people with a history of illicit drug use, but there is significant clinical experience. Dr Steven Passik of Memorial Sloan Kettering Cancer Center in New York, USA, is a leading expert on treating pain in people with a history of illicit drug use. He recommends that physicians conduct an individual risk assessment, such as the Opioid Risk Tool or SOAPP (Screener and Opioid Assessment for Patients in Pain), to assess the risks of starting a patient who may have a history of illicit drug use on strong opioid analgesics. Based on the risk assessment, the physician should develop a treatment plan that ensures good pain treatment and minimizes the risk of relapse or misuse. He recommends the following precautions for patients with a history of illicit drug use:
Basu et al. describe a similar approach to treating pain in people living with HIV who have a history of substance abuse in “Pharmacological pain control for human immunodeficiency virus-infected adults with a history of drug dependence,” Journal of Substance Abuse Treatment, vol. 32 2007), pp. 399-409. |
|
Broader Palliative Care Services The insecurity is so difficult. I don’t know what’s coming. Sometimes I think I should ask someone for something and take it and die. Sleeping is good. You forget your thoughts. Better sleep than have all sorts of ideas. —Tamara Dotsenko While physical pain is often the most immediate symptom that patients with advanced cancer and other life-limiting illnesses face, many patients also experience tremendous emotional, psychological, and spiritual pain. With a number of basic and inexpensive interventions, palliative care can often provide considerable relief of these symptoms. In Ukraine, some psychosocial and spiritual services exist in hospices and hospitals with palliative care beds, but they are altogether lacking for most patients at home. The public healthcare system focuses only on the physical condition of patients. A few lucky patients receive such support from NGOs that offer home-based palliative care services. The vast majority does not. The lack of psychosocial care for patients at home is puzzling given that Ukraine’s current system of delivering pain treatment already involves nurses visiting such patients. At present, however, these nurses just administer morphine and leave; they do not provide psychosocial support to patients and their families, no matter how heavy their burden. Viktor Bezrodny, for example, told us: “The nurse would come into the corridor. I loaded the syringe… She took the empty ampoule and we parted. She did not go to the patient.” (Human Rights Watch interview with Viktor Bezrodny, April 15, 2010.) Similarly, Katerina Potapenko, the 62-year-old wife of Arkadi, a 63-year old patient with appendix cancer, told us that the nurses would come to her house at 9 p.m., clean the area of the injection, administer the shot, and leave. But the full burden of care-giving falls to her, an elderly woman who had recently suffered a heart attack herself. She told us: “I’m both doctor and nurse. I do everything [even though] I am sick myself.” (Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with Katerina and Arkadi Potapenko, April 20, 2010.) For all its inadequacies for delivering pain treatment, Ukraine’s visiting nurses system could form the basis for providing comprehensive home-based palliative care services. With some training, these visiting nurses could coach families in providing high quality home-based care, including managing of pain and other physical symptoms and addressing the psychosocial and spiritual needs of the patient. |
IV. Exploring the Causes of Untreated Pain
The World Health Organization has urged countries to adopt national or state policies that support pain relief and palliative care; enact educational programs for the public, healthcare personnel, regulators, and other relevant parties; and modify laws and regulations to improve the availability and accessibility of drugs, especially opioid analgesics.[142] It has noted that such measures, fundamental for the development of palliative care, “cost very little but can have a significant effect.”[143]
The WHO’s recommendations correspond closely with several core obligations, which countries must meet regardless of resource availability, under the right to health. The Committee on Economic, Social and Cultural Rights (CESCR), which monitors implementation of the right to health as articulated in the International Covenant on Economic, Social and Cultural Rights (ICESCR),[144] has held that countries must adopt and implement a national public health strategy and plan of action and ensure access to essential drugs as defined by the WHO.[145] It has identified providing appropriate training for health personnel as an obligation “of comparable priority.”[146]
The Ukrainian government’s failure to take sufficient steps in these three areas not only violates the right to health, it is the primary reason for problems with palliative care and pain treatment identified in previous chapters. While the government has created a significant number of palliative care beds in public hospices and hospitals, as well as an Institute of Palliative and Hospice Medicine in the Ministry of Health, it has not taken adequate steps to ensure availability of essential palliative care medicines like oral morphine, develop a system of home-based palliative care, improve instruction for healthcare workers, or address major drug regulatory problems.
Policy
To successfully address the problems described above, a concerted and coordinated effort by a broad range of governmental and other stakeholders is needed: oral morphine must be introduced, a home-based palliative care model developed, instruction for health workers revamped, and drug regulations reformed.
As a party to the International Covenant on Economic, Social and Cultural Rights, the government has the responsibility to ensure that people with life-limiting illnesses can enjoy their right to health. It thus has to take the lead in addressing the barriers that currently impede the availability of good palliative care and pain treatment.
The Ukrainian government needs to play a much more proactive role, although it has started to take some policy steps in this direction. In 2008 it established an Institute of Palliative and Hospice Medicine within the Ministry of Health and named Professor Yuri Gubsky as its head. The institute’s mandate includes developing state programs and control over their implementation; coordinating efforts to establish a network of health institutions that provide palliative care; providing organizational and methodological support to such institutions; and conducting research.[147] Tasked with developing the government’s approach to palliative care, the institute has developed a draft national palliative care concept program that was submitted to the cabinet of ministers in October 2008.[148] The draft concept was sent back to the Ministry of Health a month later for technical reasons. To date, a new draft concept program has not yet been submitted to the cabinet of ministers, leaving Ukraine without a clear plan for developing palliative care.
As a result, Ukraine’s efforts to develop palliative care have lacked cohesion, urgency, and coordination. While the government has taken a number of important steps to enhance palliative care provision in healthcare institutions, it has not done so to ensure oral morphine becomes available, or to promote home-based palliative care. While the Institute of Palliative and Hospice Medicine has started continuing medical education courses on palliative care, there have been no centralized efforts to incorporate adequate palliative care instruction into medical school curricula or to develop a palliative care treatment guideline. While Ukraine’s drug regulators have made some changes to drug regulations to improve the availability of controlled medications, they have not addressed some of the most problematic provisions.
Education of Healthcare Workers
Lack of knowledge among healthcare workers about palliative and pain treatment services is one of the biggest obstacles to palliative care in many countries around the world. A dearth of training on the topic means that many healthcare workers do not fully understand palliative care or have the skills to provide it and subscribe to a variety of myths and misconceptions about strong opioid analgesics.
Most healthcare workers interviewed were unaware or only partially aware of international best practices for pain treatment. Many doctors and nurses expressed the erroneous belief that giving patients morphine would turn them into “drug addicts”; confused physical dependence and tolerance with dependence syndrome (addiction); interpreted patient requests for more morphine as a sign of “addiction” rather than as a sign that the current dose was insufficient; believed that one dose of morphine could provide relief far beyond the four to six hours it is active; and that a maximum daily dose was appropriate.
Healthcare workers’ inadequate knowledge about palliative care and pain treatment appears to be a direct consequence of the failure of Ukraine’s medical schools, which are all public institutions, to provide sufficient instruction on pain management and palliative care for medical students. According to palliative care experts, few medical universities have introduced specific instruction on palliative care. The mandatory undergraduate curriculum in medical schools does not include any specific instruction on palliative care, and classes about pain treatment focus primarily on acute pain (post-surgical pain, for example) rather than chronic or cancer pain.[149] While the WHO pain relief ladder is briefly mentioned, it is not studied in any detail or used in practice. In pharmacology, students learn about the pharmacological characteristics of morphine rather than its use in clinical practice.
After medical school, graduates in Ukraine go through a two-year initial specialization phase and enroll into residency programs depending on their specialization. At present, only 2 of about 19 teaching institutions offer palliative care services so most doctors specializing in oncology or anesthesiology receive no practical exposure to palliative care and pain management. Even doctors specializing in oncology do not currently do rotations in hospices. As a result, the next generation of Ukrainian doctors is educated with very limited exposure to palliative care services.
At present, just two medical institutions in Ukraine offer continuing medical education courses in palliative care: the Shchupik National Medical Academy for Post-Graduate Education and the post-graduation faculty of the Ivano-Frankiivsk Medical University. Two departments of the National Academy offer such courses. In 2010 the department of palliative care of the National Academy started offering one and two-week courses on palliative care throughout the year for oncologists, general practitioners, and nurses. The courses include bedside training.[150] The department of gerontology has organized palliative care courses since December 2009. The Ivano-Frankiivsk Medical University has included forty hours of palliative care training, including clinical training in the local hospice and in its continuing medical education courses for general practitioners.[151] All general practitioners must complete post graduate education courses once every five years.
Our research also found a conspicuous absence of evidence-based resource materials on palliative care in Ukrainian. Apart from a treatment guideline on HIV and palliative care, the Ministry of Health and professional associations have not developed clinical guidelines for palliative care or pain treatment in patients with cancer and other conditions. Textbooks used in medical and nursing schools contains little information about palliative care.[152] Pharmacology textbooks used in Ukrainian medical schools are based on a Soviet-era book that contains inaccurate information about morphine dosing.[153]
All Ukraine’s medical schools are public institutions that operate under the auspices of the Ministry of Health. The government is thus clearly in a position to ensure that adequate instruction on palliative care is provided. Human Rights Watch believes that all medical students should receive basic instruction on palliative care and pain treatment. Those whose specialize in disciplines that frequently care for people with life-limiting illnesses should receive detailed instruction and exposure to clinical practice. Failure to do so will result in a violation of the right to health.
Drug Availability
Ukraine’s drug regulations are at the heart of several of the problems with palliative care and pain management identified in previous chapters. Very strict licensing requirements have made morphine unavailable in many rural areas, and the requirement that healthcare workers must directly administer morphine to patients has led to antiquated, non-evidence based pain treatment practices. But Ukraine’s drug regulations also create a significant administrative burden for healthcare workers who prescribe opioid medications and impose a very strict control regime that generates a sense of trepidation about prescribing opioid medications among many healthcare workers. These two factors likely contribute to the reluctance among many healthcare workers to prescribe these medications and an unwarranted delay in the onset of treatment for severe pain.
Under the 1961 Single Convention on Narcotic Drugs, governments must regulate the manufacture, distribution, and prescription of controlled substances to prevent their misuse. But the convention also recognizes that these substances are “indispensable for the relief of pain and suffering” and that states must make “adequate provision to ensure [their] availability … for such purposes.”[154] In the words of the International Narcotics Control Board, the body that monitors implementation of the UN drug conventions, the 1961 convention:
… establishes a dual drug control obligation: to ensure adequate availability of narcotic drugs, including opiates, for medical and scientific purposes, while at the same time preventing illicit production of, trafficking in and use of such drugs.[155]
The 1961 Single Convention on Narcotic Drugs lays out three minimum criteria that countries must observe when developing national regulations on handling controlled medications:
- Individuals must be authorized to dispense opioids by their professional license to practice or be specially licensed to do so.
- Movement of opioids may occur only between institutions or individuals so authorized under national law.
- A medical prescription is required before opioids may be dispensed to a patient.
Additionally, countries also have to keep records on the use of controlled medications.[156]
|
Impact of Drug Control on Medicine Availability: The Example of Tramadol Tramadol is a weak opioid pain medication used to treat moderate to severe pain. In Ukraine, as in most countries, it was a regular prescription medication widely used for pain management. Unlike morphine, the use of which was mired in bureaucracy around prescription, tramadol was a hassle-free pain medication that was significantly stronger than over-the-counter pain medications. Doctors could write a simple prescription and patients could buy the medication at any pharmacy. In fact, most pharmacists sold tramadol without a prescription as well. However, the easy availability of the drug had adverse consequences. Although tramadol has unpleasant side effects, many drug users started using it to mitigate the effects of withdrawal when they did not have access to other drugs. Teenagers began experimenting with tramadol at schools; for many, tramadol was their first experience with drug use. Ukraine’s law enforcement agencies became increasingly concerned about the way tramadol was being used for non-medical purposes. Instead of enforcing existing rules for prescription medications—stopping pharmacists from giving out such medications without a prescription—the government applied an increasingly restrictive prescription regime to the medication and, eventually, scheduled it as a narcotic drug in June 2008. The effect of this decision on the availability of tramadol for legitimate medical purposes has been dramatic. According to the pharmacological center of the Ministry of Health, four producers of tramadol discontinued production. Many pharmacies were no longer allowed to stock the medication because they did not have narcotics licenses. For healthcare providers, it became as problematic to prescribe tramadol as morphine, so many stopped doing so. One oncologist told Human Rights Watch: “Prescribing tramadol is such a hassle that you might as well prescribe morphine.” A chief doctor at a central district hospital said there is not one pharmacy that stocks tramadol in his entire district of some 35 thousand people. Government estimates for domestic production of tramadol show a dramatic decrease from 2008 to 2010. In 2008 the government estimated production at 19.5 and 6.5 million grams of oral and injectable tramadol for the year. In 2010, its estimate was 1.88 million grams of tramadol or almost 14 times less. |
The convention permits governments to impose additional requirements if deemed necessary.[157] However, as WHO has observed that “this right must be continually balanced against the responsibility to ensure opioid availability for medical purposes.”[158] In other words, regulations should not unnecessarily impede access to controlled medications. WHO has developed guidelines that governments can use to develop what it has called a “practical system” of regulating healthcare workers’ handling of controlled medications, as well as guidelines for ensuring that drug control policies are properly balanced.[159]
Ukraine’s drug regulations have a strong focus on prevention of misuse of controlled medications, with many of their provisions going far beyond what is required by the UN drug conventions. Human Rights Watch recognizes that prevention of misuse is of particular importance in countries which, like Ukraine, face major problems with illicit drug use as well as significant corruption in the healthcare sector.[160] However, our research shows clearly that some provisions in Ukraine’s drug regulations are so burdensome and have such a restrictive impact on the availability of controlled medications for legitimate medical and health purposes that they lead to violations of the right to health.
Many of the healthcare workers we interviewed for this report were also concerned about the negative impact of drug regulations on legitimate medical practice. While all supported strict regulation of opioid analgesics, many felt the current regulatory regime was excessively and unnecessarily burdensome. They said that certain aspects of the regulations strongly interfered with the delivery of adequate pain treatment services and were not necessary to prevent misuse.
Ukraine’s government has begun to address some of the problematic provisions in its drug regulations. It has created a working group on pain treatment that is responsible for reviewing drug regulations. In 2010 Ukraine adopted a new regulation, Order 11, which somewhat relaxed the requirement that healthcare workers directly administer strong opioid medications by allowing self-administration of oral medications. This change means that if Ukraine introduces oral morphine, healthcare workers will be allowed to provide them with a take-home supply. In October 2010 in meetings with Human Rights Watch and the International Renaissance Foundation, Volodymyr Tymoshenko, the head of the National Drug Control Committee and Elena Koval of the department on licit narcotics circulation of the Interior Ministry stated that they were deeply concerned about the lack of narcotics licenses among rural pharmacies and health clinics.[161] Tymoshenko said that he had raised these concerns with regional officials and encouraged them to ensure that more pharmacies obtained narcotics licenses.[162]
Licensing Requirements
Under the UN drug conventions, controlled medicines may only be handled by individuals and institutions that are licensed to do so. This means that healthcare institutions and workers need to be licensed before they can stock, prescribe, or dispense opioid analgesics. Countries may set up a special licensing procedure for healthcare institutions and workers or permission to handle opioid medications can be part of the general license to operate a healthcare institution or professional license. Countries that require a separate license for institutions or healthcare workers should ensure that licensing requirements and procedures are transparent and efficient and do not create barriers to the availability and accessibility of these essential medications.
In Ukraine, healthcare institutions and pharmacies must obtain a special license from the National Drug Control Committee to be allowed to handle controlled medicines like morphine. This license also specifies which staff members of the institution are authorized to handle the medications. In interviews with Human Rights Watch, health administrators generally described the procedure for obtaining these licenses as smooth and unproblematic but said that some of the requirements a healthcare provider or pharmacy must meet to be able to get the license are problematic for many.[163] Health clinics known as feldshersko-akusherski punkty are ineligible to get a narcotics license.[164]
Ukraine’s regulations set out a number of criteria that a healthcare institution must meet before a license can be issued (summarized in Table 7).[165] Many of these requirements are significantly stricter than what is required by the UN drug conventions or is practiced in neighboring countries like Poland or Romania but most are not unreasonable. As long as they do not have an unjustifiably restrictive impact on the availability of controlled medicines for healthcare purposes, they are consistent with the right to health.
TABLE 7
Requirement |
Details |
Documents Required |
Qualified Personnel |
Management of facility must include a specialist with relevant professional training. Personnel with access to controlled medications must have relevant professional training. This requirement is differentiated for different types of healthcare facilities and pharmacies, with fewer requirements for lower level facilities. |
Certified copies of qualifications of management and personnel with access to the controlled medications. |
No Counter Indications for Personnel |
Personnel with access to controlled medicines may not have a mental disorder related to drug or alcohol abuse; may not have been declared ineligible to handle narcotics; may not have a criminal record related to illicit drugs and certain types of other criminal offenses. |
Personnel must obtain relevant certificates from state drug treatment clinics and police once per year. |
Appropriate Material Conditions |
The facilities must be such that secure and safe conditions can be created for keeping and accounting for narcotics. |
The Ministry of Interior must conduct an inspection at the site and issue a permit certifying that the premises meet requirements. |
Appropriate Sanitary Conditions |
Premises must meet the requirements of Ukraine’s sanitary norms and rules for storing of narcotics. |
Conclusion from the State Sanitary-Epidemic Service. |
Legal entity |
The healthcare provider must be a legal entity. |
|
But the requirements for storage premises (see Table 8) are highly problematic.[166] Most notably in practical terms for rural health clinics and pharmacies is the need for an alarm system. While there is no legal requirement that the alarm system be hooked up to the local police department, doctors at several rural hospitals said that this was a requirement in practice, and that the recurring monthly cost of such system, which one doctor put at 1400 hryvna (US$175), was too great for many clinics.
TABLE 8
Requirements for premises used for operations with narcotic drugs |
|
Hospitals, pharmacies |
Health clinics (ambulatoria) |
Location of storage |
|
Must be a separate room located in a “capital building.” |
Walls |
Walls must be equivalent in strength to a cement wall of a width of no less than 500 mm |
No special requirements for the walls of the room. |
Floors and ceilings |
Floors and ceilings must be equivalent in their strength to a reinforced concrete plate no less than 180 mm wide |
No special requirements for the floor/ceiling of the room. |
|
|
If above requirements for walls, floors and ceilings are not met, the entire area of the walls, floor, and ceiling must be reinforced from the inside with steel bars of no less than 10 mm in diameter, and the size of openings no more than 150 x 150 mm. The bars must be welded to the walls or plates that are clear of laying and covered by anchors with diameter a no less than 12 mm and with a step of 500 x 500 mm. Where it is impossible to install anchors, fittings made of steel strips may be embedded with dimensions of 100 x 50 x 6 mm are attached to reinforced concrete surfaces with four dowels. |
|
|
Entrance doors
|
Entrance doors must be durable, well fitted to the door frame; metal or wooden "full-body;" no less than 40 mm wide; must have two built-in, non-self-locking locks. |
Entrance doors must be durable, well fitted to the door frame; metal or wooden "full-body"; no less than 40 mm wide; must have two built-in, non-self-locking locks. |
Windows |
Window openings must be equipped with metal bars from inside or between frames; it is permissible to use decorative bars or blinds with the strengths no less than that of the metal bars. |
Window openings must be equipped with metal bars from inside or between frames; it is permissible to use decorative bars or blinds with the strengths no less than that of the metal bars. |
Storage locker |
No special requirements. |
The premise must be equipped with vaults or metal boxes attached to the floor (walls). |
Alarm system |
The premise must be equipped with an alarm system that protects potential entry routs: window and door openings, ventilation routs, heat inputs, and other elements of the premise accessible for ingress from outside; the doors must be blocked for opening and breaking; the windows must be protected against opening and breaking of the window glass; non-capital walls, ceilings, places of service lines entry must be protected against breaking; capital walls, ventilation boxes must be protected against collapse and breaking force; the alarm signal must be transmitted to the board of centralized monitoring of a department of internal affairs. |
The premise must be equipped with an autonomous alarm system that protects the inside space and surfaces of the premise, vaults (metal boxes) that are used for storage, and an alarm signal that transmits to the board of centralized monitoring or to local sound or light signaling devices. |
|
Other requirements
|
Premises, vaults and metal boxes:
|
Premises, vaults, and metal boxes:
|
These requirements are the primary reason for the limited availability of opioid analgesics in rural clinics described in Chapter II. Healthcare workers at all central district hospitals we visited told us how problematic these requirements are for rural clinics and pharmacies. The chief doctor in district 3, for example, said:
We currently pay 1400 hryvna [about US$175] per month for [an alarm system at] one facility. [In this district, we have] 12 ambulatoria plus the central district hospital. You can calculate [the cost if all health clinics had narcotics licenses]…. It is just not rational.[167]
To outfit the room, the walls have to be a certain size, these kinds of bars, such a safe that is attached [to the floor], a door that is reinforced, and an alarm system. You know, we could build additional walls of the right width, change the bars if the railing isn’t adequate. But installing an alarm system for three ampoules and hook it up to the [police] point…
We have regulations that do not differentiate whether it’s a FAP, what quantities will be stored…. This is how thick the walls must be. This is how thick the bars have to be. It is nonsense to think that someone is going to try to get into the ambulatoria, saw through the bars, open the safe, to get three ampoules…. But we have one law for all. No matter whether it’s the central district hospital or a FAP.[168]
The requirements for storage premises pursue a legitimate aim, preventing theft of controlled substances from medical channels. But they have such a restrictive impact on the availability of these medications that they do not balance the competing interest of availability with drug control. As such they are inconsistent with the right to health and the principle of balance articulated in the UN drug conventions. Ukraine needs to urgently amend these requirements.
Prescribing Procedures
The 1961 Single Convention on Narcotics Drugs contains two simple requirements for dispensing opioid analgesics to patients: they can be dispensed only on a medical prescription, and a record must be kept. The convention allows governments to impose additional requirements “if deemed necessary or desirable,” such as requiring that all prescription be written on official forms provided by the government or authorized professional associations.[169] However, as the WHO has observed, “this right must be continually balanced against the responsibility to ensure opioid availability for medical purposes.”[170] The WHO Expert Committee on Cancer Pain Relief and Active Support Care has, however, observed that special multiple-copy prescription requirements “typically … reduce prescribing of covered drugs by 50 percent or more.”[171]
Ukraine’s drug regulations provide for some of the most complex and burdensome prescription procedures for opioid analgesics in the world. While in most countries a qualified medical doctor can independently prescribe morphine whenever he or she considers it appropriate, doctors in Ukraine can do so only for up to three days. Any prescription beyond that requires a decision by “Commission on Soundness of Prescription of Narcotic Drugs,” which consists of three doctors from the institution. The treating physician must prepare a detailed written conclusion regarding the need for opioid analgesics, which the commission uses as the basis for making its decision. If the commission decides opioid analgesics are in order, the chief or a deputy chief doctor of the health facility must approve the commission’s decision before the medications can be provided to the patient.[172] For any changes in dosage of the medication, the commission has to be reconvened.
Healthcare workers we interviewed consistently told us that prescribing morphine is a time consuming process that takes anywhere from 30 minutes to 2 hours. Although most said that this does not discourage them from prescribing the medication, a doctor at a polyclinic in Kharkiv said that while he supported strict regulation “to a certain extent patients do suffer from that strictness.”[173] Based on its research, Human Rights Watch believes that the complexity of the prescribing procedure creates a barrier to the timely initiation of treatment with morphine for patients with pain.
While Ukraine’s prescription procedures may pursue a legitimate aim—preventing theft and diversion of controlled medications—they are excessively cumbersome and an impractical use of limited medical resources. Medically, involving four doctors in prescribing opioid analgesics is unnecessary. In most patients, managing cancer pain is not especially complicated, no more so than many other cancer-related health problems about which oncologists in Ukraine are allowed to make decisions independently. Indeed, it is standard practice in most countries around the world for individual doctors to make decisions regarding prescriptions of opioid analgesics. A Human Rights Watch survey of barriers to palliative care in 40 countries across the world found that only 2 countries surveyed, Russia and Ukraine, required multiple doctors to sign off on morphine prescriptions.[174] From a drug control perspective, the prescription procedure also seems excessively burdensome. While Human Rights Watch is aware of allegations of corruption in Ukraine’s healthcare sector, it should be possible to prevent corruption with less burdensome regulations.
When doctors write prescriptions to be filled at pharmacies, which few doctors do, they must do so on a special prescription form, popularly known as “the red form.”[175] These forms must be signed and stamped with the personal seal of the prescribing doctor and of the health care establishment and must also be signed by the chief doctor of the health care establishment or the deputy responsible for medical matters. The prescription must be filled within 5 days of its issuance (ordinary prescriptions in Ukraine must be filled with 30 days). A maximum of 20 ampoules of morphine can be prescribed per prescription form (see also Pharmacies and Opioid Analgesics).
Dispensing Procedures
Ukraine’s drug regulations require that injectable opioid analgesics from hospital stock must be administered to patients directly by a healthcare worker even if the patient is at home.[176] This requirement is the single most problematic provision of Ukraine’s drug regulations.
Having nurses administer morphine directly may allow the healthcare system to monitor the use of the medication very closely and prevent misuse, but nothing in the UN drug conventions requires this level of control. This system interferes with good medical care, results in significant patient suffering, and is therefore not consistent with the requirements of the drug conventions or the right to health.
This level of control is also unnecessary. All European Union countries, as well as Ukraine’s other neighbors, allow patients to take home supplies of morphine and other strong opioid analgesics.[177] For the limited numbers of cases where a real risk of misuse exists, prescribing doctors and administering nurses should be responsible for taking measures to minimize that risk, monitor the patient closely, and act promptly if there is a suspicion that medications are not being used as prescribed (see, for example, text box on “Treating Patients in Pain with a History of Illicit Drug Use”, p 54).[178]
Several doctors interviewed said that they felt that the control measures were excessive. The oncologist at district 3, for example, said:
The [level of] control is unfounded. It is purely theoretical [that people would start selling morphine]. It is far from practice. The patients we have really need it. The whole family sees that. They do everything [they can] to lighten the condition of the patient. Therefore, why would they sell them? Those who encountered this among their own relatives will not sell it. You see your suffering relative—you’re not going to take the morphine yourself [or sell it].[179]
Healthcare workers at the central district hospital in district 4, which does not comply with the requirement of direct administration and gives patients a three-day supply of morphine to take home and administer themselves, told us that in multiple years of giving patients or relatives injectable morphine to take home, they have never encountered evidence of misuse. They said that relatives faithfully return empty morphine ampoules to the clinic when they pick up their next supply. In rare cases that relatives drop an ampoule, healthcare workers said that they had brought back the broken pieces.[180]
The requirement is also unnecessary from a medical point of view. Patients in Ukraine and elsewhere routinely administer other injectable medications, such as insulin, themselves. There is no reason why, with adequate instruction from healthcare workers, relatives cannot do the same with morphine, particularly if it is administered subcutaneously. Indeed, Ukraine’s regulations allow patients to administer injectable morphine themselves if they obtain it on a prescription from a pharmacy. However, as noted above, very few doctors in Ukraine write such prescriptions.
Record Keeping
Under the 1961 Single Convention on Narcotic Drugs, governments must require hospitals and other institutions that handle opioid medications to keep “such records as will show the quantities … of each individual acquisition and disposal of drugs.”[181] These records must be preserved for no less than two years. The convention does not specify what kind of records must be kept, but an authoritative commentary states that “any usual form of recording business information in an orderly fashion would be permitted, not only in books, but also in card files.”[182]
In Ukraine, healthcare workers document almost literally every single movement of every single morphine ampoule. Nurses showed us an array of journals in which they signed for a bewildering range of transactions. The fact that morphine has been prescribed is not just recorded in the patient’s file but also in a separate journal on opioid medications by the senior nurse, who signs a journal at the pharmacy when she picks up the day’s supply of morphine ampoules for her department; the nurse who administers the morphine, who signs a journal when she picks up the ampoules, signs a second journal to indicate that she has administered the ampoule, and then a third when she returns the empty ampoule to the senior nurse.[183] Finally, every ten days, the Commission on the Destruction of Empty Ampoules, comprised of three hospital staff, including the chief or deputy chief doctor of the institution, count and dispose of empty ampoules and sign a report confirming how many ampoules were discarded.[184]
Healthcare workers, in particular the nurses responsible for maintaining the various journals, told us that they take these record keeping procedures very seriously. For many, they appeared to be a source of anxiety. Several nurses told us they regularly recount all the ampoules, afraid that there might be discrepancies. Others told us that any small errors in the records could lead to significant problems in case of an inspection. Several healthcare workers in different regions mentioned the problems they might face if the serial number of an ampoule was accidentally wiped. In that case, they said, it could not be certified that the empty ampoule was the same as the one that was given out. The oncologist in district 4 said:
Before a nurse draws morphine into the syringe, she has to disinfect it. But alcohol removes the blue serial number…. Those who don’t know have a big problem. Once people know, they know what to do [to avoid removing the number].[185]
|
Wasting Resources in a Resource Poor Healthcare System Lack of adequate funding is a major problem for Ukraine’s public healthcare system. Health worker salaries are low; buildings housing hospital and clinics often in disrepair; and patients often have to pay for medications and other health services that are supposed to be free. Yet, as this report demonstrates, Ukraine spends significant resources, both financial and personnel, on procedures with opioid analgesics, some of which are medically unnecessary and are of questionable utility as drug control measures. As noted, the direct administration of morphine by healthcare workers to patients in their homes is medically not necessary and interferes with good medical practice. But it does require significant resources. The chief doctor of a city polyclinic told us his clinic employs four nurses and four drivers and maintains two cars for the sole purpose to delivering pain medications to patients. A nurse at the same facility said: We have a special car that does nothing else but deliver narcotics. We have a special room for the nurses, a room to rest. It has a couch and a safe. They cannot leave the clinic any time of the day because there may be delivery. At eight the shift changes and key and documents are handed from one nurse to the next. On the day we visited, the nurse said that there were seven patients receiving opioid analgesics who needed shots at 6 a.m., 7 a.m., 9 a.m., 12 a.m., 6 p.m., 8 p.m., 10 p.m., 11 p.m. and midnight. This occupied the nurses for the entire day. If the seven patients received an average of two injections that day, the total work output of the two nurses and two drivers would be fourteen injections. In many places, ambulances are involved in delivering morphine injections, taking time away from emergency response situations that ambulances are meant to respond to. For example, in district 3, ambulances service the whole district with morphine injections. On many days, it makes half a dozen to a dozen trips, often to remote places, just to inject morphine. But the system also draws on the time of the doctors who prescribe opioid medications and the nurses who are responsible for record keeping. As mentioned, doctors estimated that preparing documents for a single prescription of morphine takes 30 minutes to 2 hours. The chief nurse at one central district hospital told us it takes her an average of two hours every day to hand out morphine ampoules, receive empty ones, keep the records and pick up a new supply from the pharmacy. |
Staff at central district hospital in district 4, which gives patients a three-day supply to take home, told Human Rights Watch that it specifically instructs relatives to be careful not to wipe out the serial number on the ampoules: “We warn patients to be careful.”[1]
While the Single Convention allows countries to decide what record keeping system to put in place, Ukraine’s system seems both wasteful of scarce healthcare resources (see text box above) and likely to contribute to a reluctance to prescribe opioid analgesics, both because of the time drain it represents for healthcare workers and fear that potential mistakes in the maze of record keeping requirements could lead to investigation and potentially administrative or even criminal sanctions. It is questionable how much this complex accounting system actually contributes to its rationale: preventing diversion. The Ukrainian government should explore a simpler accounting system that does not interfere with good medical practice or waste resources.
Inspections
Under Ukrainian law, a large array of government agencies has the right to conduct inspections of healthcare institutions that use opioid medications. The National Drug Control Committee, the licensing agency, conducts both routine and surprise inspections.[186] The police and prosecutor’s office can conduct inspections when they receive information about potential misuse of controlled medications. The Ministry of Health and various other health agencies also conduct inspections of healthcare institutions regarding the use of opioid medications.
While some healthcare workers we interviewed said that their institutions had not been inspected in several years, others complained that the regularity of such inspections created a significant burden for staff. They often also expressed considerable apprehension about the checks. For example, the chief doctor in district 3 said that his hospital has faced repeated inspections from various different government agencies related to the use of opioid analgesics in the last year, including the narcotics committee, the province’s health department, the pharmacological inspection, the prosecutor’s office, the state security department, and the police department. He complained that there did not appear to be any coordination between these different agencies:
They come and say: “It’s your turn. We haven’t been with you for a long time.” [I say:] “But all the others have just been.” [They say:] “Have those been? Ok, show us the documents.”[187]
An oncologist at a polyclinic in Kharkiv, which had also faced multiple inspections in the last year, told Human Rights Watch:
We are afraid. If we put a comma somewhere wrong, we have an ocean of problems. Thank God we haven't had any situation in our hospital or the area that someone sold narcotics [illegally] or didn't prescribe correctly. We're very strict in that sense. There may be mechanical errors, administrative errors; in such cases, there is an administrative sanction. But we're careful. We know the system. We teach young doctors.[188]
The inspection of healthcare institutions that work with opioid analgesics is a normal government oversight function. However, government agencies should ensure such inspections are conducted in a reasonable manner so as to minimize their impact on the provision of and access to medical care. Any potential sanctions for violations of procedures should be proportionate and not affect patient access to pain medications.
Criminal Penalties for Mishandling Opioid Medications
Under the 1961 Single Convention on Narcotic Drugs, countries are required to make it a punishable offense to intentionally distribute controlled substances in violation of the convention.[189] In other words, a healthcare worker who deliberately provides people with morphine for non-medical use must face criminal sanctions. However, the convention does not require criminal sanctions for unintentional violations of the rules of handling opioid medications. Human Rights Watch believes that unintentional mistakes in handling such medications should not be a criminal offense and that acts that do not constitute criminal negligence should be subject to administrative or disciplinary oversight.
Ukraine’s criminal code—specifically the article regarding violations of the rules for handling controlled substances—does not differentiate between intentional and unintentional violations or consider the consequences of the violation (although courts do). It provides for up to three years imprisonment, other restrictions of freedom of movement for up to four years, or a fine equivalent to fifty minimum incomes for violations of the “rules of…storing, accounting, release, distribution, sale…use of narcotics, psychotropic substances.”[190] This means that nurses who make small, unintentional record keeping errors could potentially face criminal charges, as could doctors and nurses who give patients a take-home supply of morphine or leave loaded syringes of morphine at patients’ homes.
A search of Ukraine’s court registry revealed several cases of criminal prosecutions for relatively minor violations of narcotics regulations that did not appear to have led to the diversion or misuse of opioid analgesics. For example, in January 2007 a court in Dobrovody, Zbarazhski district, in western Ukraine found the chief physician at an ambulatoria guilty of failing to properly document the use of narcotic drugs and unlicensed storage of two ampoules of tramadol. It imposed a fine of 680 hryvna (approximately US$85) and put him on probation.[191] In 2007 a court in Odessa province, southern Ukraine, found a surgeon guilty of improperly documenting the medical histories and opioid prescriptions for 5 patients, imposed a fine of 510 hryvna ($64), and removed him from his post for a year.[192] In April 2010 the Velikobelozerskiy county court in Zaporozhskaya province in eastern Ukraine found a midwife guilty of violating Ukraine’s drug regulations on storage and transportation of narcotic drugs. The midwife lost the purse in which she was carrying a seven-day supply of omnopon (twenty-one ampoules), which exceeded the three-day limit. The court imposed a fine of 510 hryvna (US$64).[193] Human Rights Watch believes that use of criminal law in such cases could be considered disproportionate to the harm caused by any failure to comply with the regulations, even if the penalties imposed are relatively light and may contribute to an atmosphere of fear when it comes to prescribing opioid medications. The Ukrainian government should review these rules so that unintentional violations of the rules are no longer a criminal offense.
A December 2007 case against the deputy chief physician of the Kamensko-Dnepr Central District Hospital in eastern Ukraine illustrates the need for regulatory reform in Ukraine. In this case, the doctor had ordered narcotic drugs from the district pharmacy despite the fact the hospital did not have a narcotics license and lacked rooms that met the requirements for storage of narcotic drugs. The prosecution alleged that the hospital and its subsidiaries illegally acquired and stored morphine, omnopon, and fentanyl between 2001 and 2004 but not that any of the drugs had been used for non-medical purposes. In her defense, the doctor argued that she was initially not aware of the requirement to obtain a narcotics license and that, when she had become cognizant of the regulations, had taken steps to fulfill the licensing requirements. She said that she had continued to order the medications because “the refusal of [narcotic] drugs to patients presents a threat to patient life and health” and would violate Ukraine’s constitution. The court rejected the defense, sentenced her to a fine of 850 hryvna ($106), and removed her from her post for a year.[194]
The Role of Pharmaceutical Company Zdorovye Narodu
The pharmaceutical company Zdorovye Narodu is the only company in Ukraine that supplies morphine. As such, it plays a crucial role in ensuring that patients with pain have access to appropriate treatment. Unfortunately, it has included a number of problematic provisions in the product information it circulates with the injectable morphine ampoules it manufactures, including the very low maximum daily dose recommendation discussed in Chapter III. Human Rights Watch has unsuccessfully sought meetings with the company to discuss these issues. A written request for clarifications was not answered.
Like other medications, morphine ampoules come with an insert that explains their uses, contraindications, and side effects. Unfortunately, the morphine insert contains a range of assertions that are factually incorrect and contribute to poor pain care for patients, including:
- Maximum daily dose recommendation. The product information leaflet states: “Maximum dosage for adults in subcutaneous injection: one time – 2ml (20mg morphine), 24 hour period – 5ml (50 mg morphine).” The WHO guideline states that there is no maximum daily dose for morphine.
- Warning about drug dependence.The insertstates that“In case of repeated morphine use, a psychological and physical dependency develops quickly (in 2-14 days from the beginning of treatment).” In fact, patients do not develop psychological dependence when they take morphine on a doctor’s prescription. They do build up tolerance and physical dependence over time, which the WHO calls “a normal pharmacological response.”[195] It means that treatment with morphine should not be abruptly discontinued even if the patient no longer experiences pain; instead, the dose of morphine should be gradually decreased to minimize the risk of abstinence (withdrawal) syndrome until treatment can be ended. This inaccurate information perpetuates common misconceptions about the risk that addiction to morphine poses.
Exacerbating the impact of the erroneous information in the insert, the Ministry of Health has included the insert’s text in its authoritative reference book on pharmaceuticals, thus endorsing it.[196]
The Role of the INCB and UNODC
The International Narcotics Control Board, an independent and quasi-judicial international body, has a mandate to monitor the implementation of the 1961 Single Convention on Narcotic Drugs and other international drug conventions. This mandate requires it to monitor efforts of governments to implement provisions of the conventions related to the prevention of illicit use of controlled substances, as well as efforts to ensure their adequate availability for medical and scientific purposes. However, it appears that in the past 10 years the INCB has monitored Ukraine’s efforts related to illicit drugs in Ukraine much more closely than those aimed at ensuring availability of controlled medications.
The INCB visited Ukraine in 2008 to examine its implementation of the UN drug conventions. While representatives of the INCB say it is standard practice to raise the issue of the availability of strong opioid analgesics on country visits, the press statement it issued following the visit states that it had discussed a variety of issues related to illicit drugs but makes no mention of any discussions regarding availability of controlled medications.[197]
A search of the INCB’s last 10 annual reports found a total of 46 mentions of Ukraine. Of those mentions, 43 concern illicit drugs and drug control and just 2 relate to licit drugs. (The final mention of Ukraine is not related to either topic). In its annual report for 2008, the INCB endorsed a new Ukrainian drug control law that strengthened control of licit narcotic drugs but did not note Ukraine’s low consumption of morphine, the problems caused by its overly stringent drug regulations, or make any reference to the treaty obligation that drug control measures be balanced and ensure adequate availability of licit drugs for medical and scientific purposes.[198]
In a March 2011 letter to Human Rights Watch, the INCB stated that it raised the issue of medical availability during its 2008 mission to Ukraine. It said that it considers the level of consumption of opioid analgesics there inadequate and that the “subject of adequate availability will continue to be prominent in the Board’s dialogue with the Government of Ukraine.”[199]
The UN Office on Drugs and Crime (UNODC) has a mandate to “assist Member States in their struggle against illicit drugs, crime and terrorism.”[200] Its activities consist of helping enhance the capacity of member states to counteract illicit drugs, crime, and terrorism; conducting research and analytical work to expand the evidence base for policy and operational decisions; and assistance with development of relevant laws and regulations. While UNODC runs a significant number of programs aimed at HIV prevention among drug users, including in Ukraine, it has traditionally done little to promote drug regulations and laws that balance availability of medications with prevention of misuse.[201] This has recently started to change. In March 2011 UNODC presented a report to the Commission on Narcotic Drugs on the issue of availability of opioid analgesics. It also mentioned the issue prominently in its World Drug Report for 2009. To date, UNODC’s work in Ukraine has not focused on ensuring that drug regulations ensure the adequate availability of controlled medications.
V. The Human Rights Analysis
National Law
Ukraine's constitution guarantees health care free of charge in state institutions.[202] Ukraine's economic struggles since it gained independence in 1991 and the resulting decline in state income have led to a significant decline in state health care expenditures. Budget shortfalls, in turn, have led government healthcare facilities to levy official fees for public healthcare services, sometimes disguised as “donations” or “voluntary cost recovery.” It is not unusual for state health care providers to also demand “informal user fees” as a condition of receiving services.[203]
In 2002 Ukraine's Constitutional Court ruled that health care in state and community facilities should be provided “without preliminary, current or subsequent payments,” but stipulated that fees could be sought for health services considered beyond the limits of health care. Certain populations considered socially vulnerable (such as people with disabilities, children under six, and retired persons receiving minimum pension) are exempt from user charges or are eligible for free or reduced cost medication or other services.[204]
The Right to Health
Health is a fundamental human right enshrined in numerous international human rights instruments. The International Covenant on Economic, Social and Cultural Rights specifies that everyone has a right “to the enjoyment of the highest attainable standard of physical and mental health.”[205] The Committee on Economic, Social and Cultural Rights, the body charged with monitoring compliance with the ICESCR, has held that states must make available in sufficient quantity “functioning public health and health-care facilities, goods and services, as well as programmes,” and that these services must be accessible.
Because states have different levels of resources, international law does not mandate the kind of healthcare to be provided. The right to health is considered a right of “progressive realization.” By becoming party to the international agreements, a state agrees “to take steps … to the maximum of its available resources” to achieve the full realization of the right to health. In other words, high-income countries will generally have to provide healthcare services at a higher level than those with limited resources. But any country will be expected to take concrete and reasonable steps toward increased services, and regression, in many cases, will constitute a violation of the right to health.
However, the Committee on Economic, Social and Cultural Rights has held that certain core obligations are so fundamental that states must fulfill them. While resource constraints may justify only partial fulfillment of some aspects of the right to health, the committee has observed with respect to the core obligations that “a State party cannot, under any circumstances whatsoever, justify its non-compliance with the core obligations… which are non-derogable.” The committee has identified, among others, the following core obligations:
- To ensure the right of access to health facilities, goods, and services on a non-discriminatory basis, especially for vulnerable or marginalized groups.
- To provide essential medicines, as compiled by the World Health Organization.
- To ensure equitable distribution of all health facilities, goods, and services; and
- To adopt and implement a national public health strategy and plan of action, on the basis of epidemiological evidence, addressing the health concerns of the whole population.[206]
The committee lists the obligation to provide appropriate training for health personnel as an “obligation of comparable priority.”
Palliative Care and the Right to Health
Given that palliative care is an essential part of healthcare, the right to health requires that countries take steps to the maximum of their available resources to ensure that it is available. Indeed, the Committee on Economic, Social and Cultural Rights has called for “attention and care for chronically and terminally ill persons, sparing them avoidable pain and enabling them to die with dignity.”[207]A number of different state obligations flow from this:
- A negative obligation to refrain from enacting policies or undertaking actions that arbitrarily interfere with the provision or development of palliative care.
- A positive obligation to take reasonable steps to facilitate the development of palliative care.
- A positive obligation to take reasonable steps to ensure the integration of palliative care into existing health services, both public and private, through the use of regulatory and other powers as well as funding streams.
No Interference with Palliative Care
The Committee on Economic, Social, and Cultural Rights has stipulated that the right to health requires states to “refrain from interfering directly or indirectly with the enjoyment of the right to health.”[208] States may not deny or limit equal access for all persons, enforce discriminatory health policies, arbitrarily impede existing health services, or limit access to information about health.[209] Applied to palliative care, this obligation means that states should ensure that their drug control regulations do not unnecessarily, and therefore arbitrarily, impede the availability and accessibility of essential palliative care medications such as morphine and other opioid analgesics. A balance must be struck between preventing misuse and ensuring accessibility and availability of medicines for licit health purposes.
Facilitating the Development of Palliative Care
The right to health also includes an obligation to take positive measures that “enable and assist individuals and communities to enjoy the right to health.”[210] When applied to palliative care, this means that states should take reasonable steps in each of the three areas the WHO has identified as essential to the development of palliative care.[211] As noted in Chapter V, the three prongs of the WHO recommendation on palliative care development correspond closely with several of the core obligations under the right to health. This means that states cannot claim insufficient resources as justification for failing to take steps in each of these three areas.[212]
Ensuring Integration of Palliative Care into Health Services
The right to health requires that states take the steps necessary for the “creation of conditions which would assure to all medical service and medical attention in the event of sickness.”[213] The Committee on Economic, Social and Cultural Rights has held that people are entitled to a “system of health protection which provides equality of opportunity for people to enjoy the highest attainable level of health.”[214]In other words, health services should be available for all health conditions, including chronic or terminal illness, on an equitable basis. The committee has called for an integrated approach to the provision of different types of health services that includes elements of “preventive, curative and rehabilitative health treatment.”[215]
The Prohibition of Cruel, Inhuman, and Degrading Treatment
The right to be free of cruel, inhuman, and degrading treatment is a fundamental human right that is recognized in numerous international and regional human rights instruments.[216] Apart from prohibiting the use of torture and other cruel, inhuman, or degrading treatment or punishment, the right also creates a positive obligation for states to protect persons in their jurisdiction from such treatment.[217]
As part of this positive obligation, states have to take steps to protect people from unnecessary pain related to a health condition. As former UN special rapporteur on torture and other cruel, inhuman or degrading treatment or punishment Manfred Nowak wrote in a joint letter with UN special rapporteur on the right to health Anand Grover to the Commission on Narcotic Drugs in December 2008:
Governments also have an obligation to take measures to protect people under their jurisdiction from inhuman and degrading treatment. Failure of governments to take reasonable measures to ensure accessibility of pain treatment, which leaves millions of people to suffer needlessly from severe and often prolonged pain, raises questions whether they have adequately discharged this obligation.[218]
In a report to the Human Rights Council, Nowak later specified that, in his expert opinion, “the de facto denial of access to pain relief, if it causes severe pain and suffering, constitutes cruel, inhuman or degrading treatment or punishment.”[219]
Not every case where a person suffers from severe pain but has no access to appropriate treatment will constitute cruel, inhuman, or degrading treatment or punishment. Human Rights Watch believes that this may be the case when the following conditions are met:
- The suffering is severe and meets the minimum threshold required under the prohibition against torture and cruel, inhuman, or degrading treatment or punishment.
- The state is, or should be, aware of the level and extent of the suffering.
- Treatment is available to remove or lessen the suffering but no appropriate treatment is offered.
- The state has no reasonable justification for the lack of availability and accessibility of evidence-based pain treatment.
In such cases, states may be liable for failing to protect a person from cruel, inhuman, or degrading treatment. The failure of the Ukrainian government to take steps to ensure that the healthcare system can provide evidence-based pain treatment meets these criteria.
VI. A Way Forward: Recommendations for Immediate Implementation
Ukraine is rapidly falling behind its neighbors with palliative care development. On its Western borders, countries like Poland, Hungary, and Romania have all reformed their drug regulations, introduced training for healthcare workers, and developed increasingly well-functioning home-based and institution-based palliative care systems. On its Eastern borders, Georgia and Armenia are also making significant progress. Georgia, which recently introduced oral morphine, has partially reformed drug regulations that had many of the same problems as Ukraine’s, adopted a national palliative care strategy, and is rolling out significant palliative care training for healthcare workers. In Armenia, the introduction of oral morphine is imminent as well.
Ukraine needs to follow the example of these neighbors and move palliative care forward. It needs to urgently formulate and implement a comprehensive strategy for developing palliative care services that includes specific steps to overcome the various policy, regulatory, and educational barriers described in this report. The government should draw on the experiences of its neighbors and the expertise of WHO’s Access to Controlled Medications Programme, the European Association of Palliative Care, and other international palliative care experts. Ukraine should closely examine the Romanian and Georgian experiences with regulatory reform as potential models for its own reform efforts.
|
The Example of Georgia With a common history to Ukraine as part of the Soviet Union, Georgia has faced many of the same barriers discussed in this report. Like Ukraine, Georgia did not have oral morphine. Multiple doctors had to sign prescriptions for morphine, which could only be written for patients with a biopsy-proven cancer diagnosis. Patients at home could only get a three day (cities and regional centers) to five day (rural areas) supply of morphine at any time. Health policies did little to support the development of palliative care and pain treatment. In the last few years the Georgian government has actively sought to address these barriers. In its annual report for 2010 the International Narcotics Control Board praised Georgia for its progress (Para 103, Report of the International Narcotics Control Board on the Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes (E/INCB/2010/1/Supp.1), see: http://incb.org/pdf/annual-report/2010/en/supp/AR10_Supp_E.pdf (accessed March 14, 2011).) In 2008 the Georgian government amended healthcare laws to incorporate palliative care, providing patients with a right to palliative care on par with preventive, curative, and rehabilitative care. Georgia’s parliament adopted a national palliative care action plan. In 2009 the government introduced oral morphine in the public healthcare system, which is now available for outpatients and increasingly also for inpatients. In 2008 Georgia amended its drug regulations to eliminate the requirement that multiple doctors sign prescriptions for strong opioid analgesics. In 2010 drug regulations were further amended to allow all trained physicians, as opposed to just oncologists, to prescribe strong opioid analgesics and remove the requirement of a biopsy-confirmed diagnosis for such prescriptions. Patients and their relatives can receive a seven-day take-home supply of morphine and administer the medication themselves. Georgia has also made instruction in modern pain management available in undergraduate medical programs at state medical universities. At Tbilisi State University, it is a compulsory part of the curriculum; at three other universities it is optional. Instruction in pain management is available in post-graduate medical education in the country. For the last five years palliative care instruction has been available as part of continuing medical education. Despite this progress, significant barriers remain. Inexpensive instant-release morphine is still unavailable in Georgia; many healthcare workers have yet to have training in palliative care; and patients have to fill prescriptions for morphine at special pharmacies located in police stations which have limited opening hours. Email correspondence with Dr. Pati Dzotsenidze of the Tbilisi State University, Faculty of Medicine and the Institute for Cancer Prevention and Palliative Medicine, Department of Pain Policy, February 28, 2011. |
Below, Human Rights Watch makes two sets of recommendations. The first addresses issues that must be remedied immediately because of their profound negative impact on good patient care. The second group contains recommendations that require a certain amount of time and cannot be implemented overnight. However, we urge the government to move on these recommendations expediently as they are all critical to ensuring good palliative care availability.
To the Ukrainian Government
Immediately:
- Ensure the availability of oral morphine. Actively engage Zdorovye Narodu and other pharmaceutical companies to introduce oral morphine. The public healthcare system should carry oral morphine at all levels of care.
- Abolish the requirement that injectable morphine and other injectable strong pain medications be administered by healthcare workers to patients at home. In consultation with medical doctors, the WHO, and other relevant experts, provide new standards for take home medicine to ensure a continuous supply of pain medications. For example, in areas with a functioning delivery service, healthcare facilities could be allowed to provide patients with at least a seven-day supply to ensure a continuous supply of pain medications. In rural areas, where access to clinics with a narcotics license is problematic, healthcare facilities could be allowed to provide patients with at least a fourteen-day supply.
- Change licensing requirements for rural clinics. Requirements for narcotics licenses must be such that all rural clinics can obtain such license, including FAPs. In particular, the government should review whether imposing the requirement of a separate storage room on rural clinics is necessary and a proportionate measure to protect against misappropriation and whether a suitable safe would achieve similar results. It should ensure that health clinics can obtain a license with a simple sound and light alarm system rather than a system with a police hookup. If a policy decision is made to leave costly requirements, the state should provide adequate budget allocation for health clinics to meet those costs.
- Disseminate the WHO pain treatment guideline to all healthcare facilities. The Ministry of Health should urge all doctors to follow the guideline’s recommendations for assessing and treating pain based on accurate pharmacological principles.
- Provide in-service training on the pain treatment guidelines for doctors throughout the public health system.
The government should also, in conjunction with all relevant stakeholders, including civil society groups, undertake the following steps:
In the Area of Policy
- Develop a home-based palliative care system. Review staffing structures for healthcare facilities so that hospices and other facilities can provide home-based palliative care; provide funds to hospices to develop such services; reform the current system for delivering strong pain medications through nurse visits to patients’ homes into a palliative care delivery system.
- Develop palliative care and pain treatment guidelines. The Ministry of Health, medical colleges, palliative care providers, and relevant civil society groups should develop a palliative care and pain treatment guideline based on international best pharmacological and practice evidence. This treatment guideline should be widely disseminated among all relevant healthcare workers and form the basis for training healthcare workers on palliative care and pain management.
- Ensure palliative care integration into disease control strategies. National cancer and HIV/AIDS control programs and other relevant disease control strategies should have a robust palliative care component, list detailed steps aimed at integrating palliative care into these strategies, and provide for specific and adequate allocations of resources for palliative care development.
In the Area of Education
- Introduce palliative care instruction into medical and nursing curricula. Establish a clear standard for education in palliative care and pain treatment to ensure that all healthcare providers have at least basic training in the discipline. Healthcare providers who see large numbers of patients in need of palliative care should receive in-depth training and exposure to clinical practice.
- Exams for medical and nursing licenses should include questions about palliative care and pain management.
- Mandate rotations in palliative care. The Ministry of Health should mandate rotations in palliative care units for students of certain postgraduate programs, including oncology, geriatrics and infectious disease, to ensure clinical exposure to palliative care.
- Develop expert training centers. The Ministry of Health should develop nodal palliative care training centers in Ukraine’s geographic zones, possibly on the basis of existing hospices.
- Develop training modules. The Ministry of Health should translate key palliative care resources into Ukrainian and develop training modules for doctors, nurses, social workers, counselors, and volunteers, in cooperation with hospices, civil society groups, and international palliative medicine experts.
- Provide continued medical education. Palliative care and pain management should be included in mandatory continued education programs for all general practitioners, oncologists, infectious disease doctors, anesthesiologists, and geriatrists. Questions about palliative care and pain management should be included in exams for physicians and nurses following these courses.
In the Area of Drug Availability
Using the WHO’s assessment tool, “Ensuring Balance in Controlled Substance Policies,” Ukraine should initiate a thorough review of its drug regulations and amend them so that they ensure adequate availability of strong opioid analgesics, while also being capable of minimizing the risks of misuse that exist in Ukraine. Particular attention should be paid to the following issues:
- Licensing requirements. These requirements should be as least burdensome as possible, while providing protection against diversion and theft. In rural clinics, the government should consider whether a solid safe would generally be adequate protection for the small amounts of opioid medications they are likely to stock.
- Take-home medications. It is standard practice in many countries around the world to provide patients with a two-week to one-month take-home supply of morphine.
- Accounting procedures should be simplified to minimize waste of limited resources.
- Number of signatures per prescription should be reduced. Doctors in most countries can make individual decisions to prescribe opioid medications.
To Zdorovye Narodu
- Amend the product information for injectable morphine to bring it in line with available evidence. The maximum daily dose recommendation and inaccurate information on the risk of psychological dependence should be removed as out of line with international standards.
- Start manufacturing oral morphine. Oral morphine can be introduced through a so-called bio-waver, as no clinical trial or other costly procedures are required for its introduction.[220] Ukraine’s essential medicines list and list of medications that can be bought from state funds include morphine—without specifying the formulation—so oral morphine could be distributed through the public healthcare system.[221]
To the International Community
To the International Narcotics Control Board
- Consistently report in the annual report on the availability of controlled substances for medical and scientific purposes in countries, including on specific barriers that impede such availability.
- Raise concern about the problems with availability of opioid analgesics raised in this report in follow-up efforts to its 2008 mission to Ukraine. In particular, the INCB should request information from the government about its efforts to ensure adequate availability of controlled substances for medical and scientific purposes and about remaining barriers. Information on this correspondence should be included in subsequent annual reports.
- Establish regular contact with key palliative care leaders to ensure the INCB receives information on opioid availability barriers directly from healthcare providers.
- Offer technical support to Ukraine in reviewing and amending current drug regulations.
To the World Health Organization and UN Office on Drugs and Crime
- Raise concerns with the Ukrainian government about the problems with availability and accessibility of controlled medications identified in this report.
- Urge the government to use the WHO tool for assessing drug policies to review its regulations and offer technical assistance.
- The WHO Access to Controlled Medications Programme should offer technical assistance to the Ukrainian government on drug regulatory reform and educational barriers.
- Urge the government to implement resolution 53/4 of the Commission on Narcotic Drugs.
To the European Union
- Raise concerns about the limited availability of palliative care and pain treatment in Ukraine as part of its structured human rights dialogue and other relevant bilateral and multilateral dialogues with the Ukrainian government, including in the context of the Association Agreement preparatory process currently underway. Ensuring adequate availability of palliative care and pain treatment should feature among the benchmarks articulated for Ukraine.
- Offer financial and technical assistance to the government of Ukraine to review and amend drug regulations, develop palliative care policies, and introduce palliative care instruction for healthcare workers. Consider involving partners in the EU-funded Access to Opioid Medication in Europe (ATOME) in this assistance.[222]
- Offer funding and technical assistance for the development of Ukrainian palliative care and pain treatment guidelines.
To the Council of Europe
The Council of Europe has recommended that member states ensure the availability of palliative care.[223] However, its recommendations have, to date, not adequately addressed the significant problems that exist in Council of Europe states with regard to availability of opioid medications. To address this shortcoming:
- The Commissioner for Human Rights should take up the issue of access to pain treatment medications and palliative care more generally, as part of his work, including specifically in Ukraine.
- The Parliamentary Assembly of the Council of Europe should appoint a rapporteur to look into the question of availability of pain treatment medications and relevant laws in the Council of Europe region, including in Ukraine.
- The Committee of Ministers should encourage all Council of Europe countries to review their drug regulations using the tool WHO has developed for this purpose.[224]
To International Donors, in particular the Global Fund against AIDS, Tuberculosis and Malaria, the US and EU Governments
- Ensure that palliative care and pain management are an integral part of any programs that are funded to provide care and treatment services to people living with HIV and AIDS.
- Require that supported healthcare institutions obtain a license for morphine and other opioid analgesics and maintain an adequate stock of these medications.
- Financially support training of healthcare workers at AIDS centers and community care centers on palliative care and pain management.
Acknowledgments
Research for this report was conducted by Diederik Lohman, senior researcher with the Health and Human Rights Division of Human Rights Watch, jointly with Andrei Rakhansky of the Institute of Legal Research and Strategies in Kharkiv, Anna Kotenko of the Rivne branch and Alena Druzhinina of the Kiev branch of the All-Ukrainian Network of People Living with HIV. Diederik Lohman wrote the report. It was reviewed by Joseph Amon, director of the Health and Human Rights Division of Human Rights Watch; Rachel Denber, deputy director of the Europe and Central Asia Division of Human Rights Watch; Veronika Szente Goldston, advocacy director of the Europe and Central Asia Division of Human Rights Watch; Aisling Reidy, senior legal advisor at Human Rights Watch; and Danielle Haas, senior editor in the Program Office of Human Rights Watch. The report was also reviewed by Dr. Kathleen Foley of Memorial Sloan Kettering Cancer Center, Dr. Frank Ferris, director of international programs at the Institute for Palliative Medicine at San Diego Hospice, Kseniya Shapoval and Victoria Tymoshevska of the International Renaissance Foundation, Ludmila Andriishin of Ivano-Frankivsk hospice, and Andrei Rakhansky of the Institute of Legal Research and Strategies.
Alex Gertner, associate with the Health and Human Rights Division at Human Rights Watch, provided invaluable assistance, as did Mari Milorava-Kelman, Alla Khalitova, Olena Baev, Brooks Bono, Claudia Stoicescu, all interns with the Health and Human Rights Division. Production assistance was provided by Mignon Lamia, Grace Choi, Kathy Mills, Anna Lopriore, Veronica Matushaj, and Fitzroy Hepkins. Serhiy Dyoma translated the report into Ukrainian, and Igor Gerbich translated the report into Russian.
We are deeply grateful to the palliative care patients and their relatives in Ukraine who, despite battling serious illness, agreed to be interviewed for this report. We will use their testimonies and this report to fight for better palliative care in Ukraine so others who develop life-threatening illness—and pain and other symptoms associated with it—will not have to endure the suffering they faced. Similar gratitude goes to the many doctors, nurses, and government officials who spoke to us frankly about pain treatment practices in Ukraine. Without them, this manuscript would not have been possible.
We further thank Dr. Thomas Dzierzanowski, a palliative care physician from Poland, Dr. Pati Dzotsenidze of the Tbilisi State University, Faculty of Medicine and Institute for Cancer Prevention and Palliative Medicine, Department of Pain Policy, Dr. Eric Krakauer, director, International Programs, Harvard Medical School Center for Palliative Care, Dr. Stephen Passik, Memorial Sloan Kettering Cancer Center, Pavlo Skala of the International HIV/AIDS Alliance in Ukraine, and Viktor Serdiuk and Olga Skorina of the All-Ukrainian Council for Patients' Rights and Safety for their help with various sections of this report.
[1] WHO, “Achieving Balance in Opioid Control Policy: Guidelines for Assessment,” 2000, p. 6, http://apps.who.int/medicinedocs/en/d/Jwhozip39e/6.html.
[2] Telephone interview with Nadezhda Zukovska, December 17, 2010. All information in this section is based on this interview, except where otherwise indicated.
[3] WHO, “Achieving Balance in Opioid Control Policy: Guidelines for Assessment,” 2000, p. 1.
[4]WHO, Cancer Pain Relief, Second Edition, With a guide to opioid availability, ( Geneva: World Health Organization, 1996), p. 16.
[5] A camera crew of the Open Society Institute filmed Vlad in May 2010 for a documentary about his case.
[6] While palliative care is often associated with terminal illness, it can benefit patients with a much broader group of illnesses or health conditions. Palliative care advocates use the term “life-limiting” illness or health condition to delineate the group of patients who would benefit from the services provided by palliative care, including symptom control, pain treatment, psychosocial and spiritual support and others. A life-limiting illness or health condition is a chronic condition that limits or has the potential to limit the patient’s ability to lead a normal life and includes, among others, cancer, HIV/AIDS, dementia, heart, renal, and liver disease, and permanent serious injury.
[7] The WHO estimates that on average about 60 percent of people who die would benefit from palliative care before death. See Stjernsward and Clark, “Palliative Medicine: A Global Perspective” in Doyle et al, eds., Oxford Textbook of Palliative Medicine, 3rd edition. In Ukraine, with a population of 45.4 million and a death rate of 15.7 per 1,000 this translates to an estimated 428 thousand individuals each year who could benefit from palliative care. (US Central Intelligence Agency, The World Fact Book, 2010, https://www.cia.gov/library/publications/the-world-factbook/geos/up.html (accessed January 3, 2011)
[8] WHO Regional Office for Europe, European Mortality Database, 2005, http://apps.who.int/whosis/database/mort/table1_process.cfm (accessed February 24, 2011).
[9] Press service of the Ministry of Health of Ukraine, “Minzdrav: Sozdana Vseukrainskaia obshestvennaia organizatsia ‘Ukrainskaia liga sodeistvia razvitiu palliativnoi I khospisnoi pomoshchi (Ministry of Health: The Al-Ukrainian League for the Development of Palliative and Hospice Care Created), December 21, 2010, http://www.kmu.gov.ua/control/ru/publish/article;jsessionid=CA13DDA8611EF7B8E83F12CEC812FD47?art_id=243933605&cat_id=33695 (accessed March 29, 2011). See also: Institute of Palliative and Hospice Medicine, “Development of Palliative Care in Ukraine in 2008.” See http://www.eurochaplains.org/ukraine_pal_developm ent_08.pdf (accessed March 14, 2011).
[10] National Program for the Battle against Oncological Diseases of 2009, on file with Human Rights Watch.
[11] The European Association for Palliative Care reports that in 2005, 85 percent of patients in the Donetsk region died at home. EAPC Task Force on the development of Palliative Care in Europe, http://www.eapcnet.org/download/forPolicy/CountriesRep/Ukraine.pdf (accessed February 28, 2011). Similarly, 82 percent of cancer deaths and 86 percent of cardiovascular deaths in Ukraine occur at home. Mykhalskyy, V, “Palliative Care in Ukraine,” http://www.eapcnet.org/download/forEAPC-East/PCinUkraineReport-2002.pdf (accessed February 28, 2011).
[12]A 2010 report by the Ministry of Health for UNAIDS found neither “home-based care” nor “palliative care and treatment of common HIV-related infections” available to the majority of people in need. Ministry of Health of Ukraine, “Ukraine: National Report on Monitoring Progress towards the UNGASS Declaration of Commitment on HIV/AIDS,” 2010, p. 103, http://data.unaids.org/pub/Report/2010/ukraine_2010_country_progress_report_en.pdf (accessed February 28, 2011).
[13] International Narcotics Control Board, “Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes,” 2010, http://incb.org/pdf/annual-report/2010/en/supp/AR10_Supp_E.pdf (accessed March 29, 2011).
[14] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with chief doctor in district 5, May 12, 2010.
[15] State Statistics Committee of Ukraine, "General results of the census", 2002, http://2001.ukrcensus.gov.ua/eng/results/general (access February 24, 2011).
[16] In Ukraine, three strong opioid analgesics are used to treat moderate to severe pain: morphine, omnopon and promedol. Omnopon is a cocktail of morphine, codeine and several other substances. Promedol is a synthetic opioid. Both omnopon and prodemol are weaker than morphine.
[17] Human Rights Watch and Institute of Legal Research and Strategies interview with nurse in district 1, April 16, 2010.
[18] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996.
[19] Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: a report from the ESMO/EAPC Opioid Policy Initiative, N. I. Cherny, J. Baselga, F. de Conno and L. Radbruch, Annals of Oncology Volume 21, Issue 3 Pp. 615-626. This survey covered all European countries with the exception of Armenia, Azerbaijan, Malta and San Marino. It did not cover most Central Asian countries. Like Ukraine, Armenia and Azerbaijan do not have any oral morphine;
Several of Ukraine’s neighbors have taken active steps to develop palliative care services, including Romania, which in 2005 overhauled and replaced its restrictive drug regulations with ones that ensure good accessibility to pain medications, and Georgia, which in 2010 adopted a national palliative care policy, introduced oral morphine, and removed several key obstacles to strong opioid analgesics availability from its drug regulations. See: Reform of drug control policy for palliative care in Romania, Daniela Mosoiu MD,Karen M Ryan MA,David E Joranson MSSW,Jody P Garthwaite BA The Lancet , June 24, 2006 ( Vol. 367, Issue 9528, Pages 2110-2117 ) DOI: 10.1016/S0140-6736(06)68482-1, see at: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(06)68482-1/abstract (accessed February 24, 2011).
[20] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996.
[21] Kathleen M. Foley, et al., "Pain Control for People with Cancer and AIDS," in Disease Control Priorities in Developing Countries, 2nd ed., (New York: Oxford University Press, 2003), pp. 981-994.
[22] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 3.
[23] WHO, “National Cancer Control Programmes: Policies and Managerial Guidelines, second edition,” pp. 86-87.
[24] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 3.
[25] 1961 Single Convention on Narcotic Drugs, http://www.incb.org/pdf/e/conv/convention_1961_en.pdf; 1971 Convention on Psychotropic Substances, http://www.incb.org/pdf/e/conv/convention_1971_en.pdf, and the 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, http://www.unodc.org/pdf/convention_1988_en.pdf (accessed February 24, 2011).
[26] International HIV/AIDS Alliance in Ukraine, “ANALYTICAL REPORT based on sociological study results Estimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine in 2009,” Kiev, 2010, p. 12. http://www.aidsalliance.org.ua/ru/library/our/monitoring/pdf/indd_en.pdf (accessed March 30, 2011).
[27] Order 11 of 2010 of the Ministry of Health on the procedure of handling narcotic drugs, psychotropic substances and precursors at healthcare facilities of Ukraine.
[28]Human Rights Watch meeting with Volodymyr Tymoshenko, head of the National Drug Control Committee, Kiev, October 22, 2010.
[29]Joint letter by the UN special rapporteur on the prevention of torture and cruel, inhuman or degrading treatment or punishment, Manfred Nowak, and the UN special rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health, Anand Grover, to the Commission on Narcotic Drugs, December 2008. A copy of the letter is available at http://www.ihra.net/Assets/1384/1/SpecialRapporteursLettertoCND012009.pdf (accessed January 16, 2009).
[30] WHO, “National Cancer Control Programmes: Policies and Managerial Guidelines, second edition,” pp. 86-87.
[31] Pain is also a symptom in various other diseases and chronic conditions and acute pain is often a side-effect of medical procedures. This paper, however, focuses on pain and other symptoms due to life-limiting illnesses.
[32] C.S. Cleeland, J.L. Ladinsky, R.C. Serlin, and N.C. Thuy, “Multidimensional Measurement of Cancer Pain: Comparisons of U.S. and Vietnamese Patients,” Journal of Pain and Symptom Management , vol. 3, no. 1 (1988), pp. 23-27; C.S. Cleeland, Y. Nakamura, T.R. Mendoza, K.R. Edwards, J. Douglas, and R.C. Serlin, “Dimensions of the Impact of Cancer Pain in a Four Country Sample: New Information from Multidimensional Scaling,” Pain , vol. 67 (1996), pp. 2-3 and 267-273; R.L. Daut and C.S. Cleeland, “The prevalence and severity of pain in cancer,” Cancer, vol. 50 (1982), 1913-8; K.M. Foley, “Pain Syndromes in Patients with Cancer,” in K.M. Foley, J.J. Bonica, and V. Ventafridda, eds., Advances in Pain Research and Therapy (New York: Raven Press, 1979), pp. 59-75; K.M. Foley, “Pain Assessment and Cancer Pain Syndromes,” in D. Doyle, G.W.C Hanks, and N. MacDonald, eds., Oxford Textbook of Palliative Medicine, 2nd edition (New York: Oxford University Press, 1979), pp. 310-331; J. Stjernsward and D. Clark, “Palliative Medicine: A Global Perspective,” in D. Doyle, G.W.C. Hanks, N. Cherny, and K. Calman, eds., Oxford Textbook of Palliative Medicine, 3rd edition (New York: Oxford University Press, 2003), pp. 1199-1222.
[33] K.M. Foley, J.L. Wagner, D.E. Joranson, and H. Gelband, “Pain Control for People with Cancer and AIDS,” Disease Control Priorities in Developing Countries, 2nd edition(New York: Oxford University Press, 2006), pp. 981-994; Francois Larue et al., “Underestimation and under-treatment of pain in HIV disease: a multicentre study,” British Medical Journal, vol. 314, no. 13, 1997, http://www.bmj.com/cgi/content/full/314/7073/23 (accessed April, 2007); J. Schofferman and R. Brody, “Pain in Far Advanced AIDS,” in K.M. Foley, J.J. Bonica, and V. Ventafridda, eds., Advances in Pain Research and Therapy (New York: Raven Press, 1990), pp. 379-386; E.J. Singer, C. Zorilla, B. Fahy-Chandon, S. Chi, K. Syndulko, and W.W. Tourtellotte, “Painful Symptoms Reported by Ambulatory HIV-Infected Men in a Longitudinal Study,” Pain, vol. 54 (1993), pp. 1 and 15-19.
[34]P. Selwyn and M. Forstein, “Overcoming the false dichotomy of curative vs. palliative care for late-stage HIV/AIDS,” JAMA, vol.290 (2003), pp.806-814.
[35] See K. Green, “Evaluating the delivery of HIV palliative care services in out-patient clinics in Viet Nam, upgrading document,” London School of Hygiene and Tropical Medicine, 2008.
[36] F. Brennan, D.B. Carr, and M.J. Cousins, “Pain Management: A Fundamental Human Right,” Anesthesia & Analgesia, vol. 105, no. 1, July 2007, pp. 205-221.
[37] O. Gureje, M. Von Korff, G.E. Simon, R. Gater, “Persistent pain and well-being: a World Health Organization study in primary care,” JAMA, vol. 280 (1998), pp. 147-151. See also B. Rosenfeld et al., “Pain in Ambulatory AIDS Patients. II: Impact of Pain on Psychological Functioning and Quality of Life,” Pain, vol. 68, no. 2-3 (1996), pp.323-328.
[38] WHO, “National Cancer Control Programme: Policies and Managerial Guidelines, second edition,” p. 83.
[39] R.L. Daut, C.S. Cleeland, and R.C. Flanery, “Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases,” Pain, vol. 17, no. 2 (1993), pp. 197-210.
[40] WHO, “Achieving Balance in Opioid Control Policy: Guidelines for Assessment,” 2000, p. 1.
[41] United Nations Economic and Social Council (ECOSOC), "Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol amending the Single Convention on Narcotic Drugs, 1961," preamble, http://www.incb.org/incb/convention_1961.html (accessed January 15, 2009).
[42] The 16th edition of WHO Model List of Essential Medicines, approved in 2010, includes the following opioid analgesics (available at: http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf, accessed February 22, 2011)
CODEINE |
Tablet: 15 mg (phosphate) [c]; 30 mg (phosphate). |
MORPHINE |
Injection: 10 mg (morphine hydrochloride or morphine sulfate) in 1‐ml ampoule. |
Oral liquid: 10 mg (morphine hydrochloride or morphine sulfate)/5 ml. |
Tablet: 10 mg (morphine sulfate). |
Tablet (prolonged release): 10 mg; 30 mg; 60 mg (morphine sulfate). |
[43] WHO Briefing Note, “Access to Controlled Medications Programme,” February 2009, http://www.who.int/medicines/areas/quality_safety/ACMP_BrNoteGenrl_EN_Feb09.pdf (accessed July 17, 2009).
[44] A 2006 literature review that compared prevalence of eleven common symptoms among patients with five advanced stage life-limiting illnesses found that studies reported depression prevalence of 3 to 77 percent in patients with advanced cancer, 10 to 82 percent in AIDS patients, 9 to 36 percent in patients with heart disease, 37 to 71 in patients with chronic obstructive pulmonary disease, and 5 to 60 in renal patients. For anxiety, reviewed studies reported prevalence of 13 to 79 percent in patients with advanced cancer, 8 to 34 percent in AIDS patients, 49 percent in patients with heart disease, 51 to 75 in patients with chronic obstructive pulmonary disease, and 39 to 70 percent in patients with renal disease. J.P. Solano, B. Gomes, I.J. Higginson, “A Comparison of Symptom Prevalence in Far Advanced Cancer, AIDS, Heart Disease, Chronic Obstructive Pulmonary Disease and Renal Disease,” Journal of Pain and Symptom Management, Vol 31 No 1 (2006).
[45]See, for example, WHO, “National Cancer Control Programme: Policies and Managerial Guidelines, second edition,” p. 83-91.
[46] Ibid., pp. 91-92.
[47] Human Rights Watch interview with Konstantin Zvarich (not his real name), April 13, 2010.
[48] Ibid.
[49] An ambulatoria is an outpatient clinic. A feldshrsko-akusherski punkt is a health point that provides basic procedures, including prenatal care and first aid. These health centers are run by feldshers, physician assistants trained in vocational medical schools and provide routine checkups, immunizations, and emergency first-aid, and midwives. There are no physicians at these clinics.
[50] Statoids, “Raions of Ukraine,” 2005, http://www.statoids.com/yua.html (accessed February 24, 2011).
[51]Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 1, April 16, 2010.
[52]Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 2, April 16, 2010.
[53] Human Rights Watch and Institute of Legal Research and Strategies interview with the chief doctor of the central district hospital in district 3, April 14, 2010.
[54] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the deputy chief doctor of the central district hospital.
[55] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with the chief doctor of the central district hospital in district 5, May 12, 2010.
[56] Correspondence with doctors in district 6, February 24, 2011.
[57] Ministry of Health Order 11 of 2010, para. 3.11.
[58]Feldshers are physician assistants trained at nursing schools and provide routine checkups, immunizations, and emergency first-aid. While some feldshers are supervised by doctors in large hospitals, many work in rural areas where they run small outpatient posts.
[59]Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 2, April 16, 2010.
[60] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with the chief doctor of the central district hospital in district 5, May 12, 2010.
[61] Human Rights Watch and Institute of Legal Research and Strategies interview with nurse in district 1, April 16, 2010.
[62] Human Rights Watch and Institute of Legal Research and Strategies interview with Yakiv Kovalenko (not his real name), April 16, 2010.
[63] Human Rights Watch and Institute of Legal Research and Strategies interview with nurse in district 1, April 16, 2010.
[64] Ibid.
[65] Ibid.
[66] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with the chief doctor of the central district hospital in district 5, May 12, 2010.
[67] Human Rights Watch and Institute of Legal Research and Strategies interview with the chief doctor of the central district hospital in district 3, April 14, 2010
[68] Ibid.
[69] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the deputy chief doctor of the central district hospital.
[70] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with a nurse of the central district hospital.
[71] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with Svitlana Bulanova (not her real name), April 21, 2010.
[72] The population of Kirovohradskaia province is 1,133,052, see: Statoids, “Regions of Ukraine”, 2005, http://www.statoids.com/yua.html (accessed March 14, 2011).
[73]Human Rights Watch and International Renaissance Foundation meeting with Volodymyr Tymoshenko, head of the National Drug Control Committee, Kiev, October 22, 2010. In follow-up conversations, Tymoshenko has told the International Renaissance Foundation that Odessa province also has only four pharmacies with narcotics licenses and that Crimea has just seven.
[74] Ministry of Health Order 11 of January 21, 2010, para. 3.8; and Ministry of Health Order 360 of July 19, 2005.
[75] A typical daily dose is 60-75 mg of oral morphine. See: Kathleen M. Foley, et al., "Pain Control for People with Cancer and AIDS," in Disease Control Priorities in Developing Countries, 2nd ed., (New York: Oxford University Press, 2003), pp. 981-994.
The equivalent for injectable morphine is indicated here.
[76] Human Rights Watch interview with Lyubov’s daughter, Olena Klochkova (not her real name), April 25, 2010. All information in this section comes from this interview unless otherwise indicated.
[77] Ibid.
[78] Ibid.
[79] Ibid.
[80]WHO, Cancer Pain Relief – With a guide to opioid availability, second edition, Geneva 1996. The WHO is currently developing on several new treatment guidelines—for chronic non-malignant pain in adults, pain related to cancer, HIV, and other progressive life-threatening illnesses in adults, and for pain in children—that will include the latest medical knowledge on pain treatment. See http://www.who.int/medicines/areas/quality_safety/Scoping_WHOGuide_non-malignant_pain_adults.pdf; http://www.who.int/medicines/areas/quality_safety/Scoping_WHOGuide_malignant_pain_adults.pdf; http://www.who.int/medicines/areas/quality_safety/Scoping_WHOGuide_chronic_pain_children.pdf (accessed February 24, 2011).
[81] O'Neill, J. F., P. A. Selwyn, and H. Schietinger, A Clinical Guide to Supportive and Palliative Care for HIV/AIDS, (Washington, DC: Health Resources and Services Administration, 2003).
[82] For the ESMO guidelines, see http://annonc.oxfordjournals.org/content/21/suppl_5/v257.full.pdf+html (accessed February 25, 2011). For the EAPC guidelines, see http://www.eapcnet.org/download/forPublications/BJC_English.pdf (accessed February 25, 2011). The American Pain Society has also published pain treatment guidelines, which can be found at: http://www.ampainsoc.org/pub/cancer.htm (accessed February 25, 2011).
[83] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p 14.
[84] UN Committee on Economic, Social and Cultural Rights, General Comment No. 14:The right to the highest attainable standard of health, November 8, 2000, para. 12. The Committee on Economic, Social and Cultural Rights is the UN body responsible for monitoring compliance with the International Covenant on Economic, Social and Cultural Rights.
[85] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 14.
[86] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p 14.
[87] The 16th edition of WHO Model List of Essential Medicines, approved in 2010, includes the following opioid analgesics (available at: http://www.who.int/medicines/publications/essentialmedicines/Updated_sixteenth_adult_list_en.pdf, accessed February 22, 2011).
[88] Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: a report from the ESMO/EAPC Opioid Policy Initiative, N. I. Cherny, J. Baselga, F. de Conno and L. Radbruch, Annals of Oncology Volume 21, Issue 3 Pp. 615-626. This survey covered all European countries with the exception of Armenia, Azerbaijan, Malta and San Marino. It did not cover most Central Asian countries. Like Ukraine, Armenia and Azerbaijan do not have any oral morphine.
[89] Correspondence with Victoria Tymoshevska, International Renaissance Foundation, March 29, 2011.
[90] Human Rights Watch interview with Olena Klochkova (not her real name), April 25, 2010.
[91] Telephone interview with Nadezhda Zukovska, December 17, 2010.
[92] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with Svitlana Bulanova (not her real name), April 21, 2010.
[93] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with a nurse of of the central district hospital.
[94] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the chief nurse at a polyclinic in Rivne, April 19, 2010; Human Rights Watch interview with doctor at hospice, April 23. 2010.
[95] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with the chief doctor of the central district hospital in district 5, May 12, 2010.
[96] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 14.
[97] Ministry of Health Order 11 of 2010, para. 3.11.
[98] Some patients we interviewed in hospices did receive morphine at least every four hours.
[99] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the chief nurse at a polyclinic in Rivne, April 19, 2010.
[100] Human Rights Watch interview with Viktor Bezrodny, April 15, 2010.
[101] Human Rights Watch and Institute of Legal Research and Strategies interview with a nurse at a polyclinic in Kharkiv, April 13, 2010.
[102] Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 3, April 14, 2010.
[103] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p 15, 16.
[104] Ibid., p. 22.
[105] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p 22.
[106] Kathleen M. Foley, et al., "Pain Control for People with Cancer and
AIDS," in Disease Control Priorities in Developing Countries, 2nd ed., (New York: Oxford University Press, 2003), pp. 981-994.
[107] Human Rights Watch interview with the chief doctor of a cancer hospital.
[108] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 27.
[109] Population figures are based on doctors’ reports or official data. See: Statoids, “Raions of Ukraine”, 2005, http://www.statoids.com/yua.html (accessed March 14, 2011).
[110] This figure includes only patients who received morphine, not those who received omnopon or promedol.
[111] Ibid.
[112] M. van den Beuken-van Everdingen, et al., “Prevalence of pain in patients with cancer: a systematic review of the past 40
years,” Annals of Oncology, vol. 18, no.9, Mar. 12, 2007, pp. 1437-1449.
[113]Human Rights Watch and Institute of Legal Research and Strategies interview with a doctor at a polyclinic in Kharkiv, April 13, 2010.
[114] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with a doctor at a hospital in Rivne, April 21, 2010.
[115] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 19.
[116] Ibid., pp. 19-20.
[117] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 16.
[118] Ibid., p. 20.
[119] Manufacturer’s product information, on file with Human Rights Watch. For conversion rates for different kinds and formulations of opioid analgesics, see: International Palliative Care Resource Center, “Education in Palliative and End-of-life Care for Oncology”, Module 2 Cancer Pain Management, Clinical Guide for Changing Opioid Analgesics, Page M2-17, 2005, http://www.ipcrc.net/epco/EPEC-O%20M02%20Pain/EPEC-O%20M02%20Pain%20PH.pdf (accessed February 24, 2011).
[120] Human Rights Watch interview with Viktor Bezrodny, April 15, 2010.
[121] Ibid.
[122] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with Roman Baranovskiy (not his real name), April 21, 2010.
[123] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with an oncologist at a hospital in Rivne, April 21, 2010. While patients who have a history of treatment with promedol or omnopon may require increased doses of morphine because they have built up some tolerance to opioids, most doctors prescribe these medications in full ampoules as well, without determining the right dose for the individual patient.
[124] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 16.
[125] Ibid., p. 22.
[126] Human Rights Watch and Institute of Legal Research and Strategies interview with a doctor at the hospice in Kharkiv, April 12, 2010. Email correspondence with Liudmila Andrishina, chief doctor of the Ivano-Frankiivsk hospice, February 25, 2011.
[127] The findings of this survey will be published in a forthcoming Human Rights Watch report on the global state of palliative care. The maximum dose in Turkey is 200 mg of oral morphine.
[128] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with a doctor at a polyclinic in Rivne, April 19, 2010.
[129] All-Ukrainian Network of People Living with HIV, Rivne branch, interview with the chief doctor of the central district hospital in district 5, May 12, 2010.
[130] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with a doctor at a polyclinic in Rivne, April 19, 2010.
[131] Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 3, April 14, 2010.
[132] Ibid.
[133] Telephone interview with Nadezhda Zukovska, December 17, 2010.
[134] Ibid.
[135] Ibid.
[136] WHO, “Cancer Pain Relief, Second Edition, with a guide to opioid availability,” 1996, p. 16.
[137] Human Rights Watch and Institute of Legal Research and Strategies interview with the chief doctor of the central district hospital in district 3, April 14, 2010.
[138] Ibid.
[139] Human Rights Watch interview with Viktor Bezrodny, April 15, 2010.
[140] UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915005090be?Opendocument (accessed May 11, 2006), para. 12b.
[141] Human Rights Watch and All-Ukrainian Council for the Rights and Safety of Patients with Natalya Malinovska, Kiev, October 20, 2010.
[142] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 3.
[143] Ibid.
[144]International Covenant on Economic, Social and Cultural Rights (ICESCR), adopted December 16, 1966, G.A. Res. 2200A (XXI), 21 U.N. GAOR Supp. (No. 16) at 49, U.N. Doc. A/6316 (1966), 993 U.N.T.S. 3, entered into force January 3, 1976, art. 11; also in the Convention on the Rights of the Child (CRC), G.A. res. 44/25, annex, 44 U.N. GAOR Supp. (No. 49) at 167, U.N. Doc. A/44/49 (1989), entered into force September 2, 1990, art. 12.
[145]UN Committee on Economic, Social and Cultural Rights, “Substantive Issues Arising in the Implementation of the International Covenant on Economic, Social and Cultural Rights,” General Comment No. 14, The Right to the Highest Attainable Standard of Health, E/C.12/2000/4 (2000), http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/40d009901358b0e2c1256915005090be?Opendocument (accessed May 11, 2006), para. 43.
[146] Ibid., para 44(f).
[147] Ministry of Health Order 159-0, July 24, 2008.
[148] On file with Human Rights Watch.
[149] Email correspondence with Ludmila Andrishina, January 5, 2011; correspondence with Olesya Bratyun, executive director of the Al-Ukrainian League for the Development of Palliative and Hospice Care, April 8, 2011.
[150]Letter from Yuri Gubski, the head of the department of palliative and hospice care of the National Medical Academy for Continuing Education, to chief doctors of healthcare institutions, December 24, 2010. The letter is on file with Human Rights Watch.
[151] Email correspondence with Liudmila Andrishina, chief doctor of Ivano-Frankivsk hospice, February 24, 2011.
[152] Ibid.
[153] Mashkovsky, M. D., Lekarstvennye sredstva [Pharmaceuticals] (Moscow: Meditsina,1984).
[154] Preamble of the 1961 Single Convention on Narcotic Drugs, https://www.incb.org/convention_1961.html; and INCB, “Availability of Opiates for Medical Needs: Report of the International Narcotics Control Board for 1995,” p. 14, http://www.incb.org/pdf/e/ar/1995/suppl1en.pdf (accessed September 25, 2009).
[155] INCB. Availability of Opiates for Medical Needs: Report of the International Narcotics Control Board for 1995. Vienna: INCB. 1995, p. 1, http://www.incb.org/pdf/e/ar/1995/suppl1en.pdf (accessed January 15, 2009).
[156] 1961 Single Convention on Narcotic Drugs, art. 34(b).
[157] Ibid., art. 30(2bii).
[158] WHO, Cancer Pain Relief, Second Edition, With a guide to opioid availability, 1996, p. 9.
[159] Ibid., p. 10. See also: WHO, WHO Policy Guidelines Ensuring Balance in National Policies on Controlled Substances, Guidance for Availability and Accessibility for Controlled Medicines, 2011, http://www.who.int/medicines/areas/quality_safety/guide_nocp_sanend/en/index.html (accessed March 29, 2011).
[160] USAID, “Corruption Assessment: Ukraine Final Report,” February 10, 2006, http://ukraine.usaid.gov/lib/evaluations/AntiCorruption.pdf (accessed March 14, 2011); Markovska, Anna, Isaeva, Anna, “Public Sector Corruption: Lessons to be learned from the Ukrainian Experience,” Crime Prevention and Community Safety, 2007, http://www.palgrave-journals.com/cpcs/journal/v9/n2/full/8150036a.html (accessed March 14, 2011); and Gorodnichenko, Yuriy, Sabirianova Peter, Klara, “Public Sector Pay and Corruption: measuring Bribery from Micro Data,” Journal of Public Economics, June 2007, vol. 91(5-6), pages 963-991.
[161] Human Rights Watch meeting with Volodymyr Tymoshenko, head of the National Drug Control Committee, Kiev, October 22, 2010. Human Rights Watch meeting with Elena Koval, section on licit narcotics circulation of the Ministry of Interior, October 22, 2010.
[162]Human Rights Watch meeting with Volodymyr Tymoshenko, head of the National Drug Control Committee, Kiev, October 22, 2010.
[163] Licenses are issued within ten days of submitting the application with all relevant documentation for a five-year period. Article 11 of the Law on Narcotic Substances, Psychotropic Substances and Precursors of February 15, 1999 (as amended on December 22, 2006), Directive of the Cabinet of Ministers of Ukraine “On Approval of the List of Documents that Must Be Added to the Application for License for Certain Types of Economic Activities No. 756 of July 4, 2001.
[164] Ministry of Health Order 356, the predecessor to Order 11, stated specifically that FAPs could receive opioid medications. That provision has been dropped from Order 11 so FAPs are no longer identified as health institutions that can obtain a narcotics license.
[165] Article 11 of the Law on Narcotic Substances, Psychotropic Substances and Precursors of February 15, 1999 (as amended on December 22, 2006), Directive of the Cabinet of Ministers of Ukraine “On Approval of the List of Documents that Must Be Added to the Application for License for Certain Types of Economic Activities No. 756 of July 4, 2001.
[166] Ministry of Internal Affairs Order 216 of May 15, 2009 on the “Requirements to Objects and Premises Designated for Conducting Activity related to Circulation of Narcotic Drugs, Psychotropic Substances, Precursors, and Storing of such Drugs and Substances Seized from Illegal Circulation”; Ministry of Health Order 11 of January 21, 2010.
[167] Human Rights Watch and Institute of Legal Research and Strategies interview with the chief doctor of the central district hospital in district 3, April 14, 2010.
[168] Ibid.
[169] 1961 Single Convention on Narcotic Drugs, art. 30(2bii).
[170] WHO, Cancer Pain Relief, Second Edition, With a guide to opioid availability, 1996, p. 9.
[171] Ibid.
[172] Ministry of Health Order 11 of January 21, 2010, para. 3.8; and Ministry of Health Order 360 of July 19, 2005.
[173] Human Rights Watch and Institute of Legal Research and Strategies interview with a chief doctor at a polyclinic in Kharkiv, April 13, 2010.
[174]The findings of this survey will be published in a forthcoming Human Rights Watch report on the global state of palliative care.
[175] Ministry of Health Order 11 of January 21, 2010, para. 2.11.2.
[176] Ministry of Health Order 11 of 2010, para. 3.11.
[177] Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: a report from the ESMO/EAPC Opioid Policy Initiative, N. I. Cherny, J. Baselga, F. de Conno and L. Radbruch, Annals of Oncology Volume 21, Issue 3 Pp. 615-626; Human Rights Watch survey of barriers to palliative care, publication forthcoming.
[178] In the United States, the Federation of State Medical Boards has developed a guideline on pain treatment that outlines the responsibilities of medical doctors related both to the provision of pain management and the prevention of misuse of opioid medications. These include, among others, careful evaluation of patients, periodic review of treatment plan, keeping of accurate and complete medical records and compliance with controlled substances regulations, http://www.medsch.wisc.edu/painpolicy/domestic/model.htm (accessed February 24, 2011).
[179] Human Rights Watch and Institute of Legal Research and Strategies interview with the district oncologist in district 3, April 14, 2010.
[180] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the head nurse of the central district hospital in district 4, April 21, 2010.
[181] 1961 Single Convention on Narcotic Drugs, art 34(2).
[182] See: http://www.drugtext.org/library/legal/treat/commentary/default.htm (accessed February 24, 2011).
[183] Article 34 of the Law on Narcotic Drugs, Psychotropic Substances and Precursors; Section 16 and Addendum 5 to Directive 589 of June 3, 2009 of the Cabinet of Ministers on the “Order of Conducting Activity Related to Turnover of Narcotic Drugs, Psychotropic Substances and Precursors”; and para. 3.13 and 3.14 of the Ministry of Health Order 11 of January 21, 2010.
[184] Ministry of Health Order 11 of January 21, 2010, para. 1.10 and 1.11.
[185] Human Rights Watch and Rivne Branch of All-Ukrainian Network of People Living with HIV interview with the deputy chief doctor of the central district hospital in district 4, April 21, 2010.
[186] Order on the State Committee of Ukraine on Drugs Control adopted by Directive of the Cabinet of Ministry No. 676 of July 28, 2010; Section 45 of the Order on Conducting Activity Related to Circulation of Narcotics, Psychotropic Substances and Precursors, and Controlling of their Circulation, adopted by Directive of the Cabinet of Ministry No. 589 of July 03, 2009.
[187] Human Rights Watch and Institute of Legal Research and Strategies interview with the chief doctor of the central district hospital in district 3, April 14, 2010.
[188] Human Rights Watch and Institute of Legal Research and Strategies interview with a chief doctor at a polyclinic in Kharkiv, April 13, 2010.
[189] 1961 Single Convention on Narcotic Drugs, art 36(1a).
[190] Article 320(1) of Ukraine’s criminal code. The provision also provides for a three-year ban on certain types of employment and activities. In cases where the violation of the rules on handling controlled substances led to large quantities of missing narcotic drugs or where a person used their official position to steal, embezzle, or misappropriate narcotic drugs, the offense is punishable by the fine of up to the equivalence of 70 minimum incomes or three to five years of imprisonment, with prohibition to occupy certain employment positions or perform certain activity for up to 3 years (Article 320(2)).
[191] See: http://www.reyestr.court.gov.ua/Review/4003114 (accessed March 18, 2011).
[192] See: http://www.reyestr.court.gov.ua/Review/444550 (accessed March 18, 2011).
[193] See: http://www.reyestr.court.gov.ua/Review/11466588 (accessed March 18, 2011).
[194] See: http://www.reyestr.court.gov.ua/Review/5166603 (accessed March 18, 2011).
[195] WHO, “Cancer Pain Relief, Second Edition, With a guide to opioid availability,” 1996, p. 16.
[196] Ministry of Health, State Formulary of Pharmaceutical Substances and Ensuring their Accessibility, January 28, 2010, http://www.moz.gov.ua/ua/portal/dn_20100128_59.html (accessed February 26, 2011).
[197] See http://www.incb.org/incb/activities.html (accessed February 23, 2011).
[198] INCB, Report of the INCB for 2008, ID number, http://www.incb.org/incb/annual-report-2008.html (accessed February 24, 2011), para. 702.
[199] Letter from Jonathan Lucas, secretary of the International Narcotics Control Board, to Joseph Amon, Human Rights Watch, March 16, 2011.
[200] See http://www.unodc.org/unodc/en/about-unodc/index.html?ref=menutop (accessed February 24, 2011).
[201] In fact, UNODC’s own model drug laws are not based on the principle of balance. See: the Model Law on the Classification of Narcotic Drugs, Psychotropic Substances and Precursors and on the Regulation of the Licit Cultivation, Production, Manufacture and Trading of Drugs; the Model Regulation Establishing an Interministerial Commission for the Coordination of Drug Control; and the Model Drug Abuse Bill, http://www.unodc.org/unodc/en/legaltools/Model.html (accessed January 24, 2009); A detailed analysis of provisions regarding controlled medications in the model laws and regulations can be found in a January 2009 report by the Pain & Policy Studies Group, entitled “Do International Model Drug Control Laws Provide for Drug Availability?” UNODC has recognized this problem and is planning on making the necessary changes to its model laws.
[202] Constitution of Ukraine, art. 49 ("The State creates conditions for effective medical service accessible to all citizens. State and communal health protection institutions provide medical care free of charge; the existing network of such institutions shall not be reduced.").
[203] Valeria Lekhan et al., Health Care Systems in Transition. Ukraine, p. 41. See also: USAID, “Corruption Assessment: Ukraine Final Report,” February 10, 2006, http://ukraine.usaid.gov/lib/evaluations/AntiCorruption.pdf (accessed March 14, 2011); Markovska, Anna, Isaeva, Anna, “Public Sector Corruption: Lessons to be learned from the Ukrainian Experience,” Crime Prevention and Community Safety, 2007, http://www.palgrave-journals.com/cpcs/journal/v9/n2/full/8150036a.html (accessed March 14, 2011); and Gorodnichenko, Yuriy, Sabirianova Peter, Klara, “Public Sector Pay and Corruption: measuring Bribery from Micro Data,” Journal of Public Economics, June 2007, vol. 91(5-6), pages 963-991.
[204] Valeria Lekhan et al., Health Care Systems in Transition. Ukraine, p. 34-40.
[205]ICESCR, art. 12.
[206] UN Committee on Economic, Social and Cultural Rights, General Comment No. 14.
[207] Ibid., para 25. While the committee included this reference in a paragraph on the right to health for older persons, the wording clearly indicates that it applies to all chronically and terminally ill persons.
[208]Ibid., para. 33.
[209]Ibid., para. 33.
[210] Ibid.,para. 37.
[211] WHO, Cancer Pain Relief Second Edition, With a Guide to Opioid Availability (Geneva: WHO Press, 1996), p. 3.
[212] UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, para 47.
[213]ICESCR, art. 12 (2).
[214] UN Committee on Economic, Social and Cultural Rights, General Comment No. 14, para 8.
[215] Ibid., para. 25.
[216]International Covenant on Civil and Political Rights (ICCPR), adopted December 16, 1966, G.A. Res. 2200A (XXI), 21 U.N. GAOR Supp. (No. 16) at 52, U.N. Doc. A/6316 (1966), 999 U.N.T.S. 171, entered into force March 23, 1976. Article 7 provides, “No one shall be subjected to torture or to cruel, inhuman or degrading treatment or punishment.” See also Universal Declaration of Human Rights (UDHR), adopted December 10, 1948, G.A. Res. 217A(III), U.N. Doc. A/810 at 71 (1948); Convention against Torture and Other Cruel, Inhuman or Degrading Treatment or Punishment (Convention against Torture), adopted December 10, 1984, G.A. res. 39/46, annex, 39 U.N. GAOR Supp. (No. 51) at 197, U.N. Doc. A/39/51 (1984), entered into force June 26, 1987; Inter-American Convention to Prevent and Punish Torture, O.A.S. Treaty Series No. 67, entered into force February 28, 1987; European Convention for the Prevention of Torture and Inhuman or Degrading Treatment or Punishment (ECPT), signed November 26, 1987, E.T.S. 126, entered into force February 1, 1989; African [Banjul] Charter on Human and Peoples’ Rights, adopted June 27, 1981, OAU Doc. CAB/LEG/67/3 rev. 5, 21 I.L.M. 58 (1982), entered into force October 21, 1986.
[217]UN Human Rights Committee, General Comment 20, para. 8, http://www.unhchr.ch/tbs/doc.nsf/(Symbol)/6924291970754969c12563ed004c8ae5?Opendocument (accessed August 29, 2009). See also the judgment of the European Court of Human Rights in Z v United Kingdom (2001) 34 EHHR 97.
[218] Joint letter by the UN special rapporteur on the prevention of torture and cruel, inhuman or degrading treatment or punishment, Manfred Nowak, and the UN special rapporteur on the right of everyone to the enjoyment of the highest attainable standard of physical and mental health, Anand Grover, to the Commission on Narcotic Drugs, December 2008. A copy of the letter is available at http://www.ihra.net/Assets/1384/1/SpecialRapporteursLettertoCND012009.pdf (accessed January 16, 2009).
[219]Human Rights Council, Report of the Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment, Manfred Nowak, A/HRC/10/44, January 14, 2009, http://daccessdds.un.org/doc/UNDOC/GEN/G09/103/12/PDF/G0910312.pdf?OpenElement (accessed August 4, 2009), para. 72.
[220] Human Rights Watch interview with Olga Baulia, State Expert Center of the Ministry of Health, October 21, 2010.
[221] Cabinet of Ministers Order 333 of March 25, 2009.
[222] For a description of the ATOME project, see: http://www.atome-project.eu/project.php (accessed February 25, 2011).
[223]Recommendation Rec (2003)24 of the Committee of Ministers to member states on the organisation of palliative care, Adopted November 12, 2003, http://www.coe.int/t/dg3/health/Source/Rec(2003)24_en.pdf (accessed February 25, 2011); Recommendation 1418 (1999) on the protection of the human rights and dignity of the terminally ill and the dying, Adopted June 25, 1999, http://assembly.coe.int/documents/adoptedtext/ta99/erec1418.htm (accessed February 25, 2011); and Parliamentary Assembly of the Council of Europe, Social, Health and Family Affairs Committee, “Palliative care: a model for innovative health and social policies,” Doc. 11758, November 4, 2008, http://assembly.coe.int/Documents/WorkingDocs/Doc08/EDOC11758.pdf (accessed February 25, 2011).
[224] WHO, WHO Policy Guidelines Ensuring Balance in National Policies on Controlled Substances, Guidance for Availability and Accessibility for Controlled Medicines, 2011, http://www.who.int/medicines/areas/quality_safety/guide_nocp_sanend/en/index.html (accessed March 29, 2011).